A multimodal iPSC platform for cystic fibrosis drug testing
- PMID: 35906215
- PMCID: PMC9338271
- DOI: 10.1038/s41467-022-31854-8
A multimodal iPSC platform for cystic fibrosis drug testing
Abstract
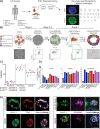
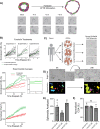
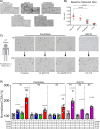
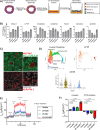
Cystic fibrosis is a monogenic lung disease caused by dysfunction of the cystic fibrosis transmembrane conductance regulator anion channel, resulting in significant morbidity and mortality. The progress in elucidating the role of CFTR using established animal and cell-based models led to the recent discovery of effective modulators for most individuals with CF. However, a subset of individuals with CF do not respond to these modulators and there is an urgent need to develop novel therapeutic strategies. In this study, we generate a panel of airway epithelial cells using induced pluripotent stem cells from individuals with common or rare CFTR variants representative of three distinct classes of CFTR dysfunction. To measure CFTR function we adapt two established in vitro assays for use in induced pluripotent stem cell-derived airway cells. In both a 3-D spheroid assay using forskolin-induced swelling as well as planar cultures composed of polarized mucociliary airway epithelial cells, we detect genotype-specific differences in CFTR baseline function and response to CFTR modulators. These results demonstrate the potential of the human induced pluripotent stem cell platform as a research tool to study CF and in particular accelerate therapeutic development for CF caused by rare variants.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Modeling Cystic Fibrosis Using Pluripotent Stem Cell-Derived Human Pancreatic Ductal Epithelial Cells.Stem Cells Transl Med. 2016 May;5(5):572-9. doi: 10.5966/sctm.2015-0276. Epub 2016 Mar 31. Stem Cells Transl Med. 2016. PMID: 27034411 Free PMC article.
-
Correlating genotype with phenotype using CFTR-mediated whole-cell Cl- currents in human nasal epithelial cells.J Physiol. 2022 Mar;600(6):1515-1531. doi: 10.1113/JP282143. Epub 2021 Dec 8. J Physiol. 2022. PMID: 34761808
-
Correction of Airway Stem Cells: Genome Editing Approaches for the Treatment of Cystic Fibrosis.Hum Gene Ther. 2020 Sep;31(17-18):956-972. doi: 10.1089/hum.2020.160. Epub 2020 Sep 8. Hum Gene Ther. 2020. PMID: 32741223 Free PMC article. Review.
-
Inflammatory Activity of Epithelial Stem Cell Variants from Cystic Fibrosis Lungs Is Not Resolved by CFTR Modulators.Am J Respir Crit Care Med. 2023 Nov 1;208(9):930-943. doi: 10.1164/rccm.202305-0818OC. Am J Respir Crit Care Med. 2023. PMID: 37695863 Free PMC article.
-
Unified Airway-Cystic Fibrosis.Otolaryngol Clin North Am. 2023 Feb;56(1):125-136. doi: 10.1016/j.otc.2022.09.009. Epub 2022 Oct 18. Otolaryngol Clin North Am. 2023. PMID: 36266104 Review.
Cited by
-
On the path to predicting immune responses in the lung: Modeling the pulmonary innate immune system at the air-liquid interface (ALI).Eur J Pharm Sci. 2023 Dec 1;191:106596. doi: 10.1016/j.ejps.2023.106596. Epub 2023 Sep 26. Eur J Pharm Sci. 2023. PMID: 37770004 Free PMC article. Review.
-
Advances in Preclinical In Vitro Models for the Translation of Precision Medicine for Cystic Fibrosis.J Pers Med. 2022 Aug 16;12(8):1321. doi: 10.3390/jpm12081321. J Pers Med. 2022. PMID: 36013270 Free PMC article. Review.
-
Modelling phenotypes, variants and pathomechanisms of syndromic diseases in different systems.Med Genet. 2024 Jun 6;36(2):121-131. doi: 10.1515/medgen-2024-2020. eCollection 2024 Jun. Med Genet. 2024. PMID: 38854643 Free PMC article.
-
Strategies to reduce the risks of mRNA drug and vaccine toxicity.Nat Rev Drug Discov. 2024 Apr;23(4):281-300. doi: 10.1038/s41573-023-00859-3. Epub 2024 Jan 23. Nat Rev Drug Discov. 2024. PMID: 38263456 Review.
-
Reconstructing the pulmonary niche with stem cells: a lung story.Stem Cell Res Ther. 2022 Apr 11;13(1):161. doi: 10.1186/s13287-022-02830-2. Stem Cell Res Ther. 2022. PMID: 35410254 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

