Restoration of Cardiomyogenesis in Aged Mouse Hearts by Voluntary Exercise
- PMID: 35862076
- PMCID: PMC9357140
- DOI: 10.1161/CIRCULATIONAHA.121.057276
Restoration of Cardiomyogenesis in Aged Mouse Hearts by Voluntary Exercise
Abstract
Background: The human heart has limited capacity to generate new cardiomyocytes and this capacity declines with age. Because loss of cardiomyocytes may contribute to heart failure, it is crucial to explore stimuli of endogenous cardiac regeneration to favorably shift the balance between loss of cardiomyocytes and the birth of new cardiomyocytes in the aged heart. We have previously shown that cardiomyogenesis can be activated by exercise in the young adult mouse heart. Whether exercise also induces cardiomyogenesis in aged hearts, however, is still unknown. Here, we aim to investigate the effect of exercise on the generation of new cardiomyocytes in the aged heart.
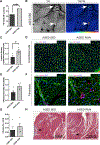
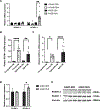
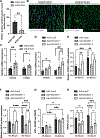
Methods: Aged (20-month-old) mice were subjected to an 8-week voluntary running protocol, and age-matched sedentary animals served as controls. Cardiomyogenesis in aged hearts was assessed on the basis of 15N-thymidine incorporation and multi-isotope imaging mass spectrometry. We analyzed 1793 cardiomyocytes from 5 aged sedentary mice and compared these with 2002 cardiomyocytes from 5 aged exercised mice, followed by advanced histology and imaging to account for ploidy and nucleation status of the cell. RNA sequencing and subsequent bioinformatic analyses were performed to investigate transcriptional changes induced by exercise specifically in aged hearts in comparison with young hearts.
Results: Cardiomyogenesis was observed at a significantly higher frequency in exercised compared with sedentary aged hearts on the basis of the detection of mononucleated/diploid 15N-thymidine-labeled cardiomyocytes. No mononucleated/diploid 15N-thymidine-labeled cardiomyocyte was detected in sedentary aged mice. The annual rate of mononucleated/diploid 15N-thymidine-labeled cardiomyocytes in aged exercised mice was 2.3% per year. This compares with our previously reported annual rate of 7.5% in young exercised mice and 1.63% in young sedentary mice. Transcriptional profiling of young and aged exercised murine hearts and their sedentary controls revealed that exercise induces pathways related to circadian rhythm, irrespective of age. One known oscillating transcript, however, that was exclusively upregulated in aged exercised hearts, was isoform 1.4 of regulator of calcineurin, whose regulation and functional role were explored further.
Conclusions: Our data demonstrate that voluntary running in part restores cardiomyogenesis in aged mice and suggest that pathways associated with circadian rhythm may play a role in physiologically stimulated cardiomyogenesis.
Keywords: age factors; circadian rhythm; exercise; heart failure; muscle development.
Figures



Similar articles
-
Exercise induces new cardiomyocyte generation in the adult mammalian heart.Nat Commun. 2018 Apr 25;9(1):1659. doi: 10.1038/s41467-018-04083-1. Nat Commun. 2018. PMID: 29695718 Free PMC article.
-
lncExACT1 and DCHS2 Regulate Physiological and Pathological Cardiac Growth.Circulation. 2022 Apr 19;145(16):1218-1233. doi: 10.1161/CIRCULATIONAHA.121.056850. Epub 2022 Feb 4. Circulation. 2022. PMID: 35114812 Free PMC article.
-
The adult heart responds to increased workload with physiologic hypertrophy, cardiac stem cell activation, and new myocyte formation.Eur Heart J. 2014 Oct 14;35(39):2722-31. doi: 10.1093/eurheartj/ehs338. Epub 2012 Oct 25. Eur Heart J. 2014. PMID: 23100284 Free PMC article.
-
Role of Mononuclear Cardiomyocytes in Cardiac Turnover and Regeneration.Curr Cardiol Rep. 2020 May 19;22(6):39. doi: 10.1007/s11886-020-01289-y. Curr Cardiol Rep. 2020. PMID: 32430578 Free PMC article. Review.
-
Polyploidy in Cardiomyocytes: Roadblock to Heart Regeneration?Circ Res. 2020 Feb 14;126(4):552-565. doi: 10.1161/CIRCRESAHA.119.315408. Epub 2020 Feb 13. Circ Res. 2020. PMID: 32078450 Review.
Cited by
-
Endothelial progenitor cell-derived extracellular vesicles: the world of potential prospects for the treatment of cardiovascular diseases.Cell Biosci. 2024 Jun 5;14(1):72. doi: 10.1186/s13578-024-01255-z. Cell Biosci. 2024. PMID: 38840175 Free PMC article. Review.
-
Realistic Aspects of Cardiac Ultrasound in Rats: Practical Tips for Improved Examination.J Imaging. 2024 Sep 6;10(9):219. doi: 10.3390/jimaging10090219. J Imaging. 2024. PMID: 39330439 Free PMC article. Review.
-
Beyond cardiomyocytes: Cellular diversity in the heart's response to exercise.J Sport Health Sci. 2023 Jul;12(4):423-437. doi: 10.1016/j.jshs.2022.12.011. Epub 2022 Dec 19. J Sport Health Sci. 2023. PMID: 36549585 Free PMC article. Review.
-
Aging in Heart Failure: Embracing Biology Over Chronology: JACC Family Series.JACC Heart Fail. 2024 May;12(5):795-809. doi: 10.1016/j.jchf.2024.02.021. Epub 2024 Apr 8. JACC Heart Fail. 2024. PMID: 38597865 Free PMC article. Review.
-
Unlocking the dark matter: noncoding RNAs and RNA modifications in cardiac aging.Am J Physiol Heart Circ Physiol. 2024 Mar 1;326(3):H832-H844. doi: 10.1152/ajpheart.00532.2023. Epub 2024 Feb 2. Am J Physiol Heart Circ Physiol. 2024. PMID: 38305752 Review.
References
-
- United Nations Department of Economic and Social Affairs U. World Population Ageing. United Nations New York. 2020. Accessed Nov 30th, 2020.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

