Summarizing Study Characteristics and Diagnostic Performance of Commercially Available Tests for Respiratory Syncytial Virus: A Scoping Literature Review in the COVID-19 Era
- PMID: 35854475
- PMCID: PMC9384538
- DOI: 10.1093/jalm/jfac058
Summarizing Study Characteristics and Diagnostic Performance of Commercially Available Tests for Respiratory Syncytial Virus: A Scoping Literature Review in the COVID-19 Era
Abstract
Background: Nonpharmaceutical interventions to prevent the spread of coronavirus disease 2019 also decreased the spread of respiratory syncytial virus (RSV) and influenza. Viral diagnostic testing in patients with respiratory tract infections (RTI) is a necessary tool for patient management; therefore, sensitive and specific tests are required. This scoping literature review aimed to summarize the study characteristics of commercially available sample-to-answer RSV tests.
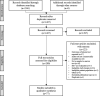
Content: PubMed and Embase were queried for studies reporting on the diagnostic performance of tests for RSV in patients with RTI (published January 2005-January 2021). Information on study design, patient and setting characteristics, and published diagnostic performance of RSV tests were extracted from 77 studies that met predefined inclusion criteria. A literature gap was identified for studies of RSV tests conducted in adult-only populations (5.3% of total subrecords) and in outpatient (7.5%) or household (0.8%) settings. Overall, RSV tests with analytical time >30 min had higher published sensitivity (62.5%-100%) vs RSV tests with analytical time ≤30 min (25.7%-100%); this sensitivity range could be partially attributed to the different modalities (antigen vs molecular) used. Molecular-based rapid RSV tests had higher published sensitivity (66.7%-100%) and specificity (94.3%-100%) than antigen-based RSV tests (sensitivity: 25.7%-100%; specificity:80.3%-100%).
Summary: This scoping review reveals a paucity of literature on studies of RSV tests in specific populations and settings, highlighting the need for further assessments. Considering the implications of these results in the current pandemic landscape, the authors preliminarily suggest adopting molecular-based RSV tests for first-line use in these settings.
Keywords: clinical; infectious disease; molecular diagnostics; nucleic-acid-based testing; point-of-care testing systems; viral diseases.
© American Association for Clinical Chemistry 2022.
Figures
Similar articles
-
Clinical performance of two commercially available rapid antigen tests for influenza, RSV, and SARS-CoV-2 diagnostics.Microbiol Spectr. 2025 Jan 7;13(1):e0163024. doi: 10.1128/spectrum.01630-24. Epub 2024 Nov 26. Microbiol Spectr. 2025. PMID: 39589150 Free PMC article.
-
Comparison of diagnostic accuracy of rapid antigen tests for COVID-19 compared to the viral genetic test in adults: a systematic review and meta-analysis.JBI Evid Synth. 2024 Oct 1;22(10):1939-2002. doi: 10.11124/JBIES-23-00291. JBI Evid Synth. 2024. PMID: 39188132 Free PMC article.
-
Antibody tests for identification of current and past infection with SARS-CoV-2.Cochrane Database Syst Rev. 2022 Nov 17;11(11):CD013652. doi: 10.1002/14651858.CD013652.pub2. Cochrane Database Syst Rev. 2022. PMID: 36394900 Free PMC article. Review.
-
Care bundles for improving outcomes in patients with COVID-19 or related conditions in intensive care - a rapid scoping review.Cochrane Database Syst Rev. 2020 Dec 21;12(12):CD013819. doi: 10.1002/14651858.CD013819. Cochrane Database Syst Rev. 2020. PMID: 33348427 Free PMC article.
-
Interventions to increase COVID-19 vaccine uptake: a scoping review.Cochrane Database Syst Rev. 2022 Aug 3;8(8):CD015270. doi: 10.1002/14651858.CD015270. Cochrane Database Syst Rev. 2022. PMID: 35920693 Free PMC article.
Cited by
-
Gold Nanourchins Improve Virus Targeting and Plasmonic Coupling for Virus Diagnosis on a Smartphone Platform.ACS Sens. 2022 Dec 23;7(12):3741-3752. doi: 10.1021/acssensors.2c01552. Epub 2022 Dec 1. ACS Sens. 2022. PMID: 36454708 Free PMC article.
-
Modeling the Transmission Mitigation Impact of Testing for Infectious Diseases.medRxiv [Preprint]. 2024 Mar 5:2023.09.22.23295983. doi: 10.1101/2023.09.22.23295983. medRxiv. 2024. Update in: Sci Adv. 2024 Jun 14;10(24):eadk5108. doi: 10.1126/sciadv.adk5108. PMID: 37808825 Free PMC article. Updated. Preprint.
-
Respiratory syncytial virus: challenges in diagnosis and impact on the elderly: Recommendations from a multidisciplinary panel.Hum Vaccin Immunother. 2024 Dec 31;20(1):2388943. doi: 10.1080/21645515.2024.2388943. Epub 2024 Aug 19. Hum Vaccin Immunother. 2024. PMID: 39161095 Free PMC article. Review.
-
Diagnostic value of routine blood tests in differentiating between SARS-CoV-2, influenza A, and RSV infections in hospitalized children: a retrospective study.BMC Pediatr. 2024 May 13;24(1):328. doi: 10.1186/s12887-024-04822-y. BMC Pediatr. 2024. PMID: 38741033 Free PMC article.
-
Impact of Nonpharmaceutical Interventions during the COVID-19 Pandemic on the Prevalence of Respiratory Syncytial Virus in Hospitalized Children with Lower Respiratory Tract Infections: A Systematic Review and Meta-Analysis.Viruses. 2024 Mar 11;16(3):429. doi: 10.3390/v16030429. Viruses. 2024. PMID: 38543794 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

