Changing global epidemiology of liver cancer from 2010 to 2019: NASH is the fastest growing cause of liver cancer
- PMID: 35793659
- PMCID: PMC9762323
- DOI: 10.1016/j.cmet.2022.05.003
Changing global epidemiology of liver cancer from 2010 to 2019: NASH is the fastest growing cause of liver cancer
Abstract
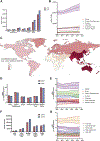
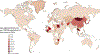
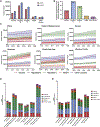
Liver cancer epidemiology is changing due to increasing alcohol consumption, rising prevalence of obesity, and advances in hepatitis B virus (HBV) and hepatitis C virus (HCV) treatment. However, the impact of these changes on global liver cancer burden remains unclear. We estimated global and regional temporal trends in the burden of liver cancer and the contributions of various liver disease etiologies using the methodology framework of the Global Burden of Disease study. Between 2010 and 2019, there was a 25% increase in liver cancer deaths. Age-standardized death rates (ASDRs) increased only in the Americas and remained stable or fell in all other regions. Between 2010 and 2019, non-alcoholic steatohepatitis (NASH) and alcohol had the fastest growing ASDRs, while HCV and HBV declined. Urgent measures are required at a global level to tackle underlying metabolic risk factors and slow the growing burden of NASH-associated liver cancer, especially in the Americas.
Keywords: alcohol; epidemiology; etiology; hepatitis B; hepatitis C; hepatocellular carcinoma; hepatoma; liver cancer; nonalcoholic steatohepatitis.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests R.L. serves as a consultant to Aardvark Therapeutics, Altimmune, Anylam/Regeneron, Amgen, Arrowhead Pharmaceuticals, AstraZeneca, Bristol-Myer Squibb, CohBar, Eli Lilly, Galmed, Gilead, Glympse Bio, Hightide, Inipharma, Intercept, Inventiva, Ionis, Janssen Inc., Madrigal, Metacrine, Inc., NGM Biopharmaceuticals, Novartis, Novo Nordisk, Merck, Pfizer, Sagimet, Theratechnologies, 89 Bio, Terns Pharmaceuticals, and Viking Therapeutics and is cofounder of LipoNexus Inc. D.Q.H. has served as an advisory board member for Eisai. A.G.S. has served as a consultant or on advisory boards for Genentech, AstraZeneca, Bayer, Eisai, Exelixis, Wako Diagnostics, Exact Sciences, Roche, Glycotest, and GRAIL.
Figures
Similar articles
-
Global burden of primary liver cancer and its association with underlying aetiologies, sociodemographic status, and sex differences from 1990-2019: A DALY-based analysis of the Global Burden of Disease 2019 study.Clin Mol Hepatol. 2023 Apr;29(2):433-452. doi: 10.3350/cmh.2022.0316. Epub 2023 Jan 4. Clin Mol Hepatol. 2023. PMID: 36597018 Free PMC article.
-
Global burden of liver cancer in males and females: Changing etiological basis and the growing contribution of NASH.Hepatology. 2023 Apr 1;77(4):1150-1163. doi: 10.1002/hep.32758. Epub 2022 Sep 12. Hepatology. 2023. PMID: 36037274
-
The changing epidemiology of adult liver transplantation in the United States in 2013-2022: The dominance of metabolic dysfunction-associated steatotic liver disease and alcohol-associated liver disease.Hepatol Commun. 2023 Dec 22;8(1):e0352. doi: 10.1097/HC9.0000000000000352. eCollection 2024 Jan 1. Hepatol Commun. 2023. PMID: 38126928 Free PMC article.
-
Changing epidemiology of hepatocellular carcinoma in Asia.Best Pract Res Clin Gastroenterol. 2015 Dec;29(6):919-28. doi: 10.1016/j.bpg.2015.09.007. Epub 2015 Sep 10. Best Pract Res Clin Gastroenterol. 2015. PMID: 26651253 Review.
-
Global epidemiology of cirrhosis - aetiology, trends and predictions.Nat Rev Gastroenterol Hepatol. 2023 Jun;20(6):388-398. doi: 10.1038/s41575-023-00759-2. Epub 2023 Mar 28. Nat Rev Gastroenterol Hepatol. 2023. PMID: 36977794 Free PMC article. Review.
Cited by
-
Optimal threshold of alpha-fetoprotein response in patients with unresectable hepatocellular carcinoma treated with atezolizumab and bevacizumab.Invest New Drugs. 2022 Dec;40(6):1290-1297. doi: 10.1007/s10637-022-01303-w. Epub 2022 Sep 24. Invest New Drugs. 2022. PMID: 36152108
-
Hepatocellular Carcinoma Chemoprevention with Generic Agents.Semin Liver Dis. 2022 Nov;42(4):501-513. doi: 10.1055/a-1942-6693. Epub 2022 Sep 14. Semin Liver Dis. 2022. PMID: 36104114 Free PMC article. Review.
-
NAFLD/MASLD and the Gut-Liver Axis: From Pathogenesis to Treatment Options.Metabolites. 2024 Jun 28;14(7):366. doi: 10.3390/metabo14070366. Metabolites. 2024. PMID: 39057689 Free PMC article. Review.
-
Attributable liver cancer deaths and disability-adjusted life years in China and worldwide: profiles and changing trends.Cancer Biol Med. 2024 Jul 16;21(8):679-91. doi: 10.20892/j.issn.2095-3941.2024.0149. Cancer Biol Med. 2024. PMID: 39015006 Free PMC article.
-
Global epidemiology of alcohol-associated cirrhosis and HCC: trends, projections and risk factors.Nat Rev Gastroenterol Hepatol. 2023 Jan;20(1):37-49. doi: 10.1038/s41575-022-00688-6. Epub 2022 Oct 18. Nat Rev Gastroenterol Hepatol. 2023. PMID: 36258033 Free PMC article. Review.
References
-
- Asrani SK, Devarbhavi H, Eaton J, and Kamath PS (2019). Burden of liver diseases in the world. J. Hepatol. 70, 151–171. - PubMed
-
- Dang H, Yeo YH, Yasuda S, Huang CF, Iio E, Landis C, Jun DW, Enomoto M, Ogawa E, Tsai PC, et al. (2020). Cure with interferon-free direct-acting antiviral is associated with increased survival in patients with hepatitis C virus-related hepatocellular carcinoma from both east and west. Hepatology 71, 1910–1922. - PubMed
-
- Desai N, Rich NE, Jain MK, Blackwell JM, Murphy CC, Perryman P, McBryde J, Quirk L, Clark C, Villarreal D, et al. (2021). Randomized clinical trial of inreach with or without mailed outreach to promote hepatitis C screening in a difficult-to-reach patient population. Am. J. Gastroenterol. 116, 976–983. - PubMed
-
- Dyson J, Jaques B, Chattopadyhay D, Lochan R, Graham J, Das D, Aslam T, Patanwala I, Gaggar S, Cole M, et al. (2014). Hepatocellular cancer: the impact of obesity, type 2 diabetes and a multidisciplinary team. J. Hepatol. 60, 110–117. - PubMed
Publication types
MeSH terms
Grants and funding
- U01 DK130190/DK/NIDDK NIH HHS/United States
- R01 CA194307/CA/NCI NIH HHS/United States
- UL1 TR001442/TR/NCATS NIH HHS/United States
- P42 ES010337/ES/NIEHS NIH HHS/United States
- P30 DK120515/DK/NIDDK NIH HHS/United States
- U01 AA029019/AA/NIAAA NIH HHS/United States
- R01 MD012565/MD/NIMHD NIH HHS/United States
- U01 DK061734/DK/NIDDK NIH HHS/United States
- P01 HL147835/HL/NHLBI NIH HHS/United States
- R01 DK121378/DK/NIDDK NIH HHS/United States
- R01 DK106419/DK/NIDDK NIH HHS/United States
- R01 CA215520/CA/NCI NIH HHS/United States
- U01 CA230694/CA/NCI NIH HHS/United States
- P30 DK056338/DK/NIDDK NIH HHS/United States
- R01 DK124318/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

