Unbalanced bidirectional radial stiffness gradients within the organ of Corti promoted by TRIOBP
- PMID: 35737845
- PMCID: PMC9245700
- DOI: 10.1073/pnas.2115190119
Unbalanced bidirectional radial stiffness gradients within the organ of Corti promoted by TRIOBP
Abstract
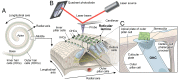
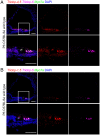
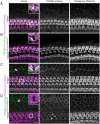
Hearing depends on intricate morphologies and mechanical properties of diverse inner ear cell types. The individual contributions of various inner ear cell types into mechanical properties of the organ of Corti and the mechanisms of their integration are yet largely unknown. Using sub-100-nm spatial resolution atomic force microscopy (AFM), we mapped the Young's modulus (stiffness) of the apical surface of the different cells of the freshly dissected P5-P6 cochlear epithelium from wild-type and mice lacking either Trio and F-actin binding protein (TRIOBP) isoforms 4 and 5 or isoform 5 only. Variants of TRIOBP are associated with deafness in human and in Triobp mutant mouse models. Remarkably, nanoscale AFM mapping revealed unrecognized bidirectional radial stiffness gradients of different magnitudes and opposite orientations between rows of wild-type supporting cells and sensory hair cells. Moreover, the observed bidirectional radial stiffness gradients are unbalanced, with sensory cells being stiffer overall compared to neighboring supporting cells. Deafness-associated TRIOBP deficiencies significantly disrupted the magnitude and orientation of these bidirectional radial stiffness gradients. In addition, serial sectioning with focused ion beam and backscatter scanning electron microscopy shows that a TRIOBP deficiency results in ultrastructural changes of supporting cell apical phalangeal microfilaments and bundled cortical F-actin of hair cell cuticular plates, correlating with messenger RNA and protein expression levels and AFM stiffness measurements that exposed a softening of the apical surface of the sensory epithelium in mutant mice. Altogether, this additional complexity in the mechanical properties of the sensory epithelium is hypothesized to be an essential contributor to frequency selectivity and sensitivity of mammalian hearing.
Keywords: actin cytoskeleton; atomic force microscopy; biophysics; hearing mechanics; mechanobiology.
Conflict of interest statement
The authors declare no competing interest.
Figures




Similar articles
-
The actin cytoskeleton in hair bundle development and hearing loss.Hear Res. 2023 Sep 1;436:108817. doi: 10.1016/j.heares.2023.108817. Epub 2023 May 26. Hear Res. 2023. PMID: 37300948 Free PMC article. Review.
-
R1 motif is the major actin-binding domain of TRIOBP-4.Biochemistry. 2013 Aug 6;52(31):5256-64. doi: 10.1021/bi400585h. Epub 2013 Jul 22. Biochemistry. 2013. PMID: 23789641
-
TRIOBP-5 sculpts stereocilia rootlets and stiffens supporting cells enabling hearing.JCI Insight. 2019 Jun 20;4(12):e128561. doi: 10.1172/jci.insight.128561. eCollection 2019 Jun 20. JCI Insight. 2019. PMID: 31217345 Free PMC article.
-
Supervillin Is a Component of the Hair Cell's Cuticular Plate and the Head Plates of Organ of Corti Supporting Cells.PLoS One. 2016 Jul 14;11(7):e0158349. doi: 10.1371/journal.pone.0158349. eCollection 2016. PLoS One. 2016. PMID: 27415442 Free PMC article.
-
The TRIOBP Isoforms and Their Distinct Roles in Actin Stabilization, Deafness, Mental Illness, and Cancer.Molecules. 2020 Oct 27;25(21):4967. doi: 10.3390/molecules25214967. Molecules. 2020. PMID: 33121024 Free PMC article. Review.
Cited by
-
Atomic force microscopy-mediated mechanobiological profiling of complex human tissues.Biomaterials. 2023 Dec;303:122389. doi: 10.1016/j.biomaterials.2023.122389. Epub 2023 Nov 11. Biomaterials. 2023. PMID: 37988897 Free PMC article. Review.
-
Combined AAV-mediated gene replacement therapy improves auditory function in a mouse model of human DFNB42 deafness.Mol Ther. 2023 Sep 6;31(9):2783-2795. doi: 10.1016/j.ymthe.2023.07.014. Epub 2023 Jul 22. Mol Ther. 2023. PMID: 37481704 Free PMC article.
-
The actin cytoskeleton in hair bundle development and hearing loss.Hear Res. 2023 Sep 1;436:108817. doi: 10.1016/j.heares.2023.108817. Epub 2023 May 26. Hear Res. 2023. PMID: 37300948 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

