Serious arrhythmia in initiators of citalopram, escitalopram, and other selective serotonin reuptake inhibitors: A population-based cohort study in older adults
- PMID: 35733364
- PMCID: PMC9468567
- DOI: 10.1111/cts.13319
Serious arrhythmia in initiators of citalopram, escitalopram, and other selective serotonin reuptake inhibitors: A population-based cohort study in older adults
Abstract
The selective serotonin reuptake inhibitors (SSRIs) citalopram and escitalopram are associated with QT prolongation, which increases the risk of serious arrhythmia. Consequently, regulatory agencies issued safety warnings in 2011. This study aimed to investigate the risk of serious arrhythmia following initiation of citalopram or escitalopram compared to other SSRIs and the risk in the periods before and after the warnings were issued. We conducted a series of nationwide cohort studies emulating a target trial using Danish healthcare register data from January 1, 2002, to December 31, 2016. We included patients (aged ≥65 years) who filled an SSRI prescription with a 1-year washout period before the index date. The outcome was an event of serious arrhythmia. Individuals were followed for a maximum of 6 months using an intention-to-treat approach. Log-binomial regression analyses were performed, estimating risk ratios (RRs) and 95% confidence intervals (CIs) adjusting for age and sex, comorbidities, and comedications with propensity scores. Dose-response effects were not investigated because dosage instructions were not available. We included 167,366 (146,014 individuals), 40,113 (37,069 individuals), and 50,281 (44,754 individuals) person-trials of citalopram, escitalopram, and other SSRIs, respectively. In total, there were 228 events of serious arrhythmia. No difference in risk was observed in the entire study period for either citalopram (0.87 [0.62-1.22]) or escitalopram (0.85 [0.53-1.40]). We identified lower point estimates after the safety warning, RR 0.54 (95% CI 0.31-0.93) for citalopram and 0.58 (0.20-1.63) for escitalopram. Initiation of citalopram and escitalopram was not associated with an increased risk of serious arrhythmia. However, lower point estimates were observed after the safety warning.
© 2022 The Authors. Clinical and Translational Science published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
At the time of the study, M.L.D.B. was an employee of the Copenhagen Centre for Regulatory Science (CORS). CORS is a cross‐faculty university‐anchored institution involving various public (Danish Medicines Agency, Copenhagen University) and private (Novo Nordisk A/S, Lundbeck A/S, Ferring pharmaceuticals A/S, LEO pharma A/S) stakeholders as well as patient organizations (Rare Diseases Denmark). The center is purely devoted to the scientific aspects of the regulatory field and has a patient‐oriented focus, and the research is not a company‐specific product or directly company related. Currently, M.L.D.B. is employed by Utrecht University to conduct research under the umbrella of the Utrecht Centre for Pharmaceutical Policy and Regulation. This center receives no direct funding or donations from private parties, including the pharma industry. Research funding from public–private partnerships (e.g., IMI, The Escher Project; http://escher.lygature.org/), is accepted under the condition that no company‐specific study is conducted. The center has received unrestricted research funding from public sources (e.g., World Health Organization, Netherlands Organization for Health Research and Development, the Dutch National Health Care Institute, EC Horizon 2020, the Dutch Medicines Evaluation Board, and the Dutch Ministry of Health). M.An. has participated in research projects funded by AstraZeneca, H. Lundbeck & Mertz, Novartis, and Pfizer, and has received fees for leading courses and teaching from Atrium, the Danish Association of the Pharmaceutical Industry. All other authors declared no competing interests for this work.
Figures
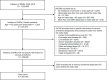
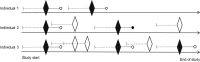
 Prescription included as a person‐trial (eligibility, exposure washout, and exclusion criteria fulfilled);
Prescription included as a person‐trial (eligibility, exposure washout, and exclusion criteria fulfilled);  Prescription not included (criteria not fulfilled); ● Outcome within 6 months after the index date; ○ Censoring (death, emigration, end of follow‐up, or end of study);
Prescription not included (criteria not fulfilled); ● Outcome within 6 months after the index date; ○ Censoring (death, emigration, end of follow‐up, or end of study);  1‐year washout for the exposure.
1‐year washout for the exposure.Similar articles
-
Selective Serotonin Reuptake Inhibitor Use and Risk of Arrhythmia: A Nationwide, Population-Based Cohort Study.Clin Ther. 2019 Jun;41(6):1128-1138.e8. doi: 10.1016/j.clinthera.2019.04.023. Clin Ther. 2019. PMID: 31178037
-
A comparative study of QT prolongation with serotonin reuptake inhibitors.Psychopharmacology (Berl). 2017 Oct;234(20):3075-3081. doi: 10.1007/s00213-017-4685-7. Epub 2017 Aug 3. Psychopharmacology (Berl). 2017. PMID: 28770276
-
Proton pump inhibitors may enhance the risk of citalopram- and escitalopram-associated sudden cardiac death among patients receiving hemodialysis.Pharmacoepidemiol Drug Saf. 2022 Jun;31(6):670-679. doi: 10.1002/pds.5428. Epub 2022 Mar 24. Pharmacoepidemiol Drug Saf. 2022. PMID: 35285107 Free PMC article.
-
[Citalopram and QT prolongation].Vnitr Lek. 2018 Winter;63(12):952-956. Vnitr Lek. 2018. PMID: 29334745 Review. Czech.
-
Escitalopram: a review of its use in the management of major depressive disorder in adults.CNS Drugs. 2010 Sep;24(9):769-96. doi: 10.2165/11204760-000000000-00000. CNS Drugs. 2010. PMID: 20806989 Review.
Cited by
-
Interpretable Machine Learning Prediction of Drug-Induced QT Prolongation: Electronic Health Record Analysis.J Med Internet Res. 2022 Dec 1;24(12):e42163. doi: 10.2196/42163. J Med Internet Res. 2022. PMID: 36454608 Free PMC article.
-
Reporting of Observational Studies Explicitly Aiming to Emulate Randomized Trials: A Systematic Review.JAMA Netw Open. 2023 Sep 5;6(9):e2336023. doi: 10.1001/jamanetworkopen.2023.36023. JAMA Netw Open. 2023. PMID: 37755828 Free PMC article.
-
Citalopram & escitalopram: Mechanisms of cardiotoxicity, toxicology predisposition and risks of use in geriatric & hemodialysis populations.Glob Cardiol Sci Pract. 2024 Aug 1;2024(4):e202434. doi: 10.21542/gcsp.2024.34. eCollection 2024 Aug 1. Glob Cardiol Sci Pract. 2024. PMID: 39351473 Free PMC article. Review.
References
-
- Chu A, Wadhwa R. Selective Serotonin Reuptake Inhibitors. In: StatPearls [internet]. StatPearls Publishing; 2021. Accessed December 7, 2021. http://www.ncbi.nlm.nih.gov/books/NBK554406/. - PubMed
-
- RADS . Behandlingsvejledning for almen praksis – Unipolar depression . 2015.
-
- Forns J, Pottegård A, Reinders T, et al. Antidepressant use in Denmark, Germany, Spain, and Sweden between 2009 and 2014: incidence and comorbidities of antidepressant initiators. J Affect Disord. 2019;249:242‐252. - PubMed
-
- Jayasinghe R, Kovoor P. Drugs and the QTc interval . Accessed December 7, 2021. https://www.nps.org.au/australian‐prescriber/articles/drugs‐and‐the‐qtc‐....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

