Nano-calcium silicate mineralized fish scale scaffolds for enhancing tendon-bone healing
- PMID: 35633872
- PMCID: PMC9123220
- DOI: 10.1016/j.bioactmat.2022.04.030
Nano-calcium silicate mineralized fish scale scaffolds for enhancing tendon-bone healing
Abstract
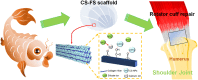
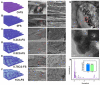
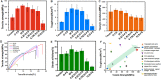
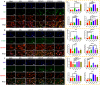
Tendon-bone healing is essential for an effective rotator cuff tendon repair surgery, however, this remains a significant challenge due to the lack of biomaterials with high strength and bioactivity. Inspired by the high-performance exoskeleton of natural organisms, we set out to apply natural fish scale (FS) modified by calcium silicate nanoparticles (CS NPs) as a new biomaterial (CS-FS) to overcome the challenge. Benefit from its "Bouligand" microstructure, such FS-based scaffold maintained excellent tensile strength (125.05 MPa) and toughness (14.16 MJ/m3), which are 1.93 and 2.72 times that of natural tendon respectively, allowing it to well meet the requirements for rotator cuff tendon repair. Additionally, CS-FS showed diverse bioactivities by stimulating the differentiation and phenotypic maintenance of multiple types of cells participated into the composition of tendon-bone junction, (e.g. bone marrow mesenchymal stem cells (BMSCs), chondrocyte, and tendon stem/progenitor cells (TSPCs)). In both rat and rabbit rotator cuff tear (RCT) models, CS-FS played a key role in the tendon-bone interface regeneration and biomechanical function, which may be achieved by activating BMP-2/Smad/Runx2 pathway in BMSCs. Therefore, natural fish scale -based biomaterials are the promising candidate for clinical tendon repair due to their outstanding strength and bioactivity.
Keywords: Bioactivities; Fish scales; High strength; Tendon repair; Tendon-bone healing enhancement.
© 2022 The Authors.
Figures

Similar articles
-
Engineered tendon-fibrocartilage-bone composite and bone marrow-derived mesenchymal stem cell sheet augmentation promotes rotator cuff healing in a non-weight-bearing canine model.Biomaterials. 2019 Feb;192:189-198. doi: 10.1016/j.biomaterials.2018.10.037. Epub 2018 Oct 29. Biomaterials. 2019. PMID: 30453215
-
Decellularized Human Umbilical Cord Wharton Jelly Scaffold Improves Tendon Regeneration in a Rabbit Rotator Cuff Tendon Defect Model.Am J Sports Med. 2022 Feb;50(2):371-383. doi: 10.1177/03635465211055722. Epub 2021 Nov 5. Am J Sports Med. 2022. PMID: 34739346
-
The 3D-Printed PLGA Scaffolds Loaded with Bone Marrow-Derived Mesenchymal Stem Cells Augment the Healing of Rotator Cuff Repair in the Rabbits.Cell Transplant. 2020 Jan-Dec;29:963689720973647. doi: 10.1177/0963689720973647. Cell Transplant. 2020. PMID: 33300392 Free PMC article.
-
Cell- and gene-based approaches to tendon regeneration.J Shoulder Elbow Surg. 2012 Feb;21(2):278-94. doi: 10.1016/j.jse.2011.11.015. J Shoulder Elbow Surg. 2012. PMID: 22244071 Review.
-
Augmentation of Rotator Cuff Repair With Soft Tissue Scaffolds.Orthop J Sports Med. 2015 Jun 10;3(6):2325967115587495. doi: 10.1177/2325967115587495. eCollection 2015 Jun. Orthop J Sports Med. 2015. PMID: 26665095 Free PMC article. Review.
Cited by
-
A New Tissue Engineering Strategy to Promote Tendon-bone Healing: Regulation of Osteogenic and Chondrogenic Differentiation of Tendon-derived Stem Cells.Orthop Surg. 2024 Oct;16(10):2311-2325. doi: 10.1111/os.14152. Epub 2024 Jul 23. Orthop Surg. 2024. PMID: 39043618 Free PMC article. Review.
-
Fabrication Strategies for Bioceramic Scaffolds in Bone Tissue Engineering with Generative Design Applications.Biomimetics (Basel). 2024 Jul 5;9(7):409. doi: 10.3390/biomimetics9070409. Biomimetics (Basel). 2024. PMID: 39056850 Free PMC article. Review.
-
Sustainable Sources of Raw Materials for Additive Manufacturing of Bone-Substituting Biomaterials.Adv Healthc Mater. 2024 Jan;13(1):e2301837. doi: 10.1002/adhm.202301837. Epub 2023 Aug 10. Adv Healthc Mater. 2024. PMID: 37535435 Free PMC article. Review.
-
Strategies to promote tendon-bone healing after anterior cruciate ligament reconstruction: Present and future.Front Bioeng Biotechnol. 2023 Mar 13;11:1104214. doi: 10.3389/fbioe.2023.1104214. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 36994361 Free PMC article. Review.
-
Strategies for promoting tendon-bone healing: Current status and prospects.Front Bioeng Biotechnol. 2023 Jan 27;11:1118468. doi: 10.3389/fbioe.2023.1118468. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 36777256 Free PMC article. Review.
References
-
- Rudzki J.R., Adler R.S., Warren R.F., Kadrmas W.R., Vermc N., Pearle A.D., Lyman S., Fealy S. Contrast-enhanced ultrasound characterization of the vascularity of the rotator cuff tendon: age- and activity-related changes in the intact asymptomatic rotator cuff. J. Shoulder Elbow Surg. 2008;17(1):96S–100S. doi: 10.1016/j.jse.2007.07.004. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

