Safety, Adherence and Persistence in a Real-World Cohort of German MS Patients Newly Treated With Ocrelizumab: First Insights From the CONFIDENCE Study
- PMID: 35614917
- PMCID: PMC9126090
- DOI: 10.3389/fneur.2022.863105
Safety, Adherence and Persistence in a Real-World Cohort of German MS Patients Newly Treated With Ocrelizumab: First Insights From the CONFIDENCE Study
Abstract
Background: Real-world relapsing multiple sclerosis (RMS) and primary progressive MS (PPMS) populations may be more diverse than in clinical trials. Here, we present a first analysis of safety, adherence and persistence data from a real-world cohort of patients newly treated with ocrelizumab.
Methods: CONFIDENCE (ML39632, EUPAS22951) is an ongoing multicenter, non-interventional post authorization safety study assessing patients with RMS or PPMS newly treated with ocrelizumab or other disease-modifying therapies for up to 10 years. For this analysis, patients newly treated with ocrelizumab were analyzed in subgroups by MS phenotype and age over a mean ~1 year of exposure totaling 2,329 patient years [PY]).
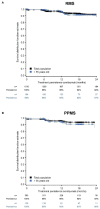
Results: At data cutoff (14 October 2020), 1,702 patients with RMS and 398 patients with PPMS were treated with ≥1 dose of ocrelizumab. At baseline, the mean ages (SD) of patients with RMS and PPMS were 41.59 (11.24) and 50.95 (9.88) years and the mean EDSS (Expanded Disability Status Scale) was 3.18 (1.87) and 4.41 (1.59), respectively. The most common adverse events (AEs) and serious AEs across both phenotypes were infections and infestations, with infection SAE rates of 2.8 events/100 PY and 1.5 events/100 PY in patients with RMS and PPMS, respectively. Across all phenotypes, ocrelizumab persistence was 92% at 24 months; median time between doses was ~6 months.
Conclusions: The ocrelizumab safety profile observed in the CONFIDENCE real-world MS population was consistent to the one observed in pivotal clinical trials. High treatment persistence and adherence were observed.
Trial registration: ML39632, EUPAS22951.
Keywords: drug (or treatment) persistence; humanized monoclonal antibody anti-CD20; multiple sclerosis; neurodegenerative diseases; non-interventional study (NIS); ocrelizumab; real-world cohort; safety.
Copyright © 2022 Weber, Buttmann, Meuth, Dirks, Muros-Le Rouzic, Eggebrecht, Hieke-Schulz, Leemhuis and Ziemssen.
Conflict of interest statement
MW receives research support from the Deutsche Forschungsgemeinschaft (DFG; WE 3547/5-1), from Novartis, TEVA, Biogen-Idec, Roche, Merck and the ProFutura Programm of the Universitätsmedizin Göttingen. MW is serving as an editor for PLoS ONE, received travel funding and/or speaker honoraria from Biogen-Idec, Merck Serono, Novartis, Roche, TEVA, Bayer and Genzyme. MB received honoraria for lecturing, consulting and/or travel expenses for attending meetings from Bayer, Biogen, Boehringer, Bristol Myers Squibb, Coloplast, Daiichi-Sankyo, Das Fortbildungskolleg, Medscape, Merck, Novartis, Roche, Sandoz, Sanofi, and Teva. SM received honoraria for lecturing and travel expenses for attending meetings from Almirall, Amicus Therapeutics Germany, Bayer Health Care, Biogen, Celgene, Diamed, Genzyme, MedDay Pharmaceuticals, Merck Serono, Novartis, Novo Nordisk, ONO Pharma, Roche, Sanofi-Aventis, Chugai Pharma, QuintilesIMS, and Teva, research is funded by the German Ministry for Education and Research (BMBF), Bundesinstitut für Risikobewertung (BfR), Deutsche Forschungsgemeinschaft (DFG), Else Kröner Fresenius Foundation, Gemeinsamer Bundesausschuss (G-BA), German Academic Exchange Service, Hertie Foundation, Interdisciplinary Center for Clinical Studies (IZKF) Muenster, German Foundation Neurology and Alexion, Almirall, Amicus Therapeutics Germany, Biogen, Diamed, Fresenius Medical Care, Genzyme, HERZ Burgdorf, Merck Serono, Novartis, ONO Pharma, Roche and Teva. PD is an employee of F. Hoffmann-La Roche AG and shareholder of F. Hoffmann-La Roche AG. JE is an employee of Roche Pharma AG and shareholder of F. Hoffmann-La Roche AG. SH-S is an employee of Roche Pharma AG. JL is an employee of Roche Pharma AG and shareholder of F. Hoffmann-La Roche AG. EM-LR is an employee of F. Hoffmann-La Roche AG and shareholder of F. Hoffmann-La Roche AG. TZ reports grants and personal fees from Biogen, Roche, TEVA and grants, personal fees from Almirall, Biogen, Celgene, Biogen, Novartis, Merck, TEVA, Janssen, Roche. The authors declare that this study received funding from Roche. The funder had the following involvement in the study: study design, analysis, interpretation of data, the writing of this article and the decision to submit it for publication.
Figures
Similar articles
-
Real-World Persistence with Ocrelizumab in Multiple Sclerosis: a Systematic Review.Neurol Ther. 2024 Oct 19. doi: 10.1007/s40120-024-00667-w. Online ahead of print. Neurol Ther. 2024. PMID: 39425889 Review.
-
Design of a non-interventional post-marketing study to assess the long-term safety and effectiveness of ocrelizumab in German real world multiple sclerosis cohorts - the CONFIDENCE study protocol.BMC Neurol. 2020 Mar 14;20(1):95. doi: 10.1186/s12883-020-01667-7. BMC Neurol. 2020. PMID: 32171264 Free PMC article.
-
Ocrelizumab: A Review in Multiple Sclerosis.Drugs. 2022 Feb;82(3):323-334. doi: 10.1007/s40265-022-01672-9. Epub 2022 Feb 22. Drugs. 2022. PMID: 35192158 Free PMC article. Review.
-
Ocrelizumab Treatment in Patients with Primary Progressive Multiple Sclerosis: Short-term Safety Results from a Compassionate Use Programme in Germany.Clin Neurol Neurosurg. 2020 Oct;197:106142. doi: 10.1016/j.clineuro.2020.106142. Epub 2020 Aug 12. Clin Neurol Neurosurg. 2020. PMID: 32920498
-
Ocrelizumab treatment in multiple sclerosis: A Danish population-based cohort study.Eur J Neurol. 2022 Feb;29(2):496-504. doi: 10.1111/ene.15142. Epub 2021 Oct 26. Eur J Neurol. 2022. PMID: 34644452
Cited by
-
Best supportive care for patients with primary progressive multiple sclerosis (PPMS) in Germany prior to ocrelizumab treatment: Final results from the RETRO PPMS study.J Cent Nerv Syst Dis. 2024 Oct 23;16:11795735241296001. doi: 10.1177/11795735241296001. eCollection 2024. J Cent Nerv Syst Dis. 2024. PMID: 39449826 Free PMC article.
-
Real-World Persistence with Ocrelizumab in Multiple Sclerosis: a Systematic Review.Neurol Ther. 2024 Oct 19. doi: 10.1007/s40120-024-00667-w. Online ahead of print. Neurol Ther. 2024. PMID: 39425889 Review.
-
Five-year efficacy outcomes of ocrelizumab in relapsing multiple sclerosis: A propensity-matched comparison of the OPERA studies with other disease-modifying therapies in real-world lines of treatments.J Cent Nerv Syst Dis. 2024 Sep 13;16:11795735241260563. doi: 10.1177/11795735241260563. eCollection 2024. J Cent Nerv Syst Dis. 2024. PMID: 39290861 Free PMC article.
-
Dermatological Neoplastic Diseases Complicating Treatment with Monoclonal Antibodies for Multiple Sclerosis.J Clin Med. 2024 Aug 29;13(17):5133. doi: 10.3390/jcm13175133. J Clin Med. 2024. PMID: 39274345 Free PMC article. Review.
-
Primary Progressive Multiple Sclerosis-A Key to Understanding and Managing Disease Progression.Int J Mol Sci. 2024 Aug 11;25(16):8751. doi: 10.3390/ijms25168751. Int J Mol Sci. 2024. PMID: 39201438 Free PMC article. Review.
References
-
- Butzkueven H, Spelman T, Ozakbas S, Kalincik T, Boz C, Buzzard K, Skibina O, Alroughani R, Real-world experience with Ocrelizumab in the MSBase Registry . Presented at ECTRIMS-ACTRIMs. (2020). 10.1136/bmjno-2021-ANZAN.10 - DOI
-
- Ziemssen T, Lang M, Tackenberg B, Schmidt S, Albrecht H, Klotz L, et al. . Clinical and demographic profile of patients receiving fingolimod in clinical practice in germany and the benefit-risk profile of fingolimod after 1 year of treatment: initial results from the observational, noninterventional study PANGAEA. Neurotherapeutics. (2018) 15:190–9. 10.1007/s13311-017-0595-y - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources