Ex Vivo Herpes Simplex Virus Reactivation Involves a Dual Leucine Zipper Kinase-Dependent Wave of Lytic Gene Expression That Is Independent of Histone Demethylase Activity and Viral Genome Synthesis
- PMID: 35604215
- PMCID: PMC9215252
- DOI: 10.1128/jvi.00475-22
Ex Vivo Herpes Simplex Virus Reactivation Involves a Dual Leucine Zipper Kinase-Dependent Wave of Lytic Gene Expression That Is Independent of Histone Demethylase Activity and Viral Genome Synthesis
Abstract
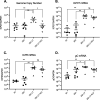
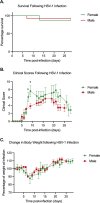
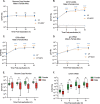
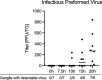
Herpes simplex virus 1 (HSV-1) maintains a lifelong latent infection in neurons and periodically reactivates, resulting in the production of infectious virus. The exact cellular pathways that induce reactivation are not understood. In primary neuronal models of HSV latency, the cellular protein dual leucine zipper kinase (DLK) has been found to initiate a wave of viral gene expression known as phase I. Phase I occurs independently of both viral DNA replication and the activities of histone demethylase enzymes required to remove repressive heterochromatin modifications associated with the viral genome. In this study, we investigated whether phase I-like gene expression occurs in ganglia reactivated from infected mice. Using the combined trigger of explant-induced axotomy and inhibition of phosphatidylinositide 3-kinase (PI3K) signaling, we found that HSV lytic gene expression was induced rapidly from both sensory and sympathetic neurons. Ex vivo reactivation involved a wave of viral late gene expression that occurred independently of viral genome synthesis and histone demethylase activity and preceded the detection of infectious virus. Importantly, we found that DLK was required for the initial induction of lytic gene expression. These data confirm the essential role of DLK in inducing HSV-1 gene expression from the heterochromatin-associated genome and further demonstrate that HSV-1 gene expression during reactivation occurs via mechanisms that are distinct from lytic replication. IMPORTANCE Reactivation of herpes simplex virus from a latent infection is associated with clinical disease. To develop new therapeutics that prevent reactivation, it is important to understand how viral gene expression initiates following a reactivation stimulus. Dual leucine zipper kinase (DLK) is a cellular protein that has previously been found to be required for HSV reactivation from sympathetic neurons in vitro. Here, we show that DLK is essential for reactivation from sensory ganglia isolated from infected mice. Furthermore, we show that DLK-dependent gene expression ex vivo occurs via mechanisms that are distinct from production replication, namely, lytic gene expression that is independent of viral DNA replication and histone demethylase activity. The identification of a DLK-dependent wave of lytic gene expression from sensory ganglia will ultimately permit the development of novel therapeutics that target lytic gene expression and prevent the earliest stage of reactivation.
Keywords: herpes simplex virus; latent infection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
DLK-Dependent Biphasic Reactivation of Herpes Simplex Virus Latency Established in the Absence of Antivirals.J Virol. 2022 Jun 22;96(12):e0050822. doi: 10.1128/jvi.00508-22. Epub 2022 May 24. J Virol. 2022. PMID: 35608347 Free PMC article.
-
Neuronal hyperexcitability is a DLK-dependent trigger of herpes simplex virus reactivation that can be induced by IL-1.Elife. 2020 Dec 22;9:e58037. doi: 10.7554/eLife.58037. Elife. 2020. PMID: 33350386 Free PMC article.
-
Evidence that the herpes simplex virus type 1 ICP0 protein does not initiate reactivation from latency in vivo.J Virol. 2006 Nov;80(22):10919-30. doi: 10.1128/JVI.01253-06. Epub 2006 Aug 30. J Virol. 2006. PMID: 16943285 Free PMC article.
-
Restarting Lytic Gene Transcription at the Onset of Herpes Simplex Virus Reactivation.J Virol. 2017 Jan 3;91(2):e01419-16. doi: 10.1128/JVI.01419-16. Print 2017 Jan 15. J Virol. 2017. PMID: 27807236 Free PMC article. Review.
-
A comparison of herpes simplex virus type 1 and varicella-zoster virus latency and reactivation.J Gen Virol. 2015 Jul;96(Pt 7):1581-602. doi: 10.1099/vir.0.000128. Epub 2015 Mar 20. J Gen Virol. 2015. PMID: 25794504 Free PMC article. Review.
Cited by
-
The Intersection of Innate Immune Pathways with the Latent Herpes Simplex Virus Genome.J Virol. 2023 May 31;97(5):e0135222. doi: 10.1128/jvi.01352-22. Epub 2023 Apr 27. J Virol. 2023. PMID: 37129520 Free PMC article. Review.
-
Co-option of mitochondrial nucleic acid sensing pathways by HSV-1 UL12.5 for reactivation from latent Infection.bioRxiv [Preprint]. 2024 Jul 9:2024.07.06.601241. doi: 10.1101/2024.07.06.601241. bioRxiv. 2024. PMID: 39005440 Free PMC article. Preprint.
-
Regulation of the Activity of the Dual Leucine Zipper Kinase by Distinct Mechanisms.Cells. 2024 Feb 11;13(4):333. doi: 10.3390/cells13040333. Cells. 2024. PMID: 38391946 Free PMC article. Review.
-
Impact of Cultured Neuron Models on α-Herpesvirus Latency Research.Viruses. 2022 Jun 2;14(6):1209. doi: 10.3390/v14061209. Viruses. 2022. PMID: 35746680 Free PMC article. Review.
-
c-Jun Signaling During Initial HSV-1 Infection Modulates Latency to Enhance Later Reactivation in addition to Directly Promoting the Progression to Full Reactivation.bioRxiv [Preprint]. 2023 Nov 10:2023.11.10.566462. doi: 10.1101/2023.11.10.566462. bioRxiv. 2023. Update in: J Virol. 2024 Feb 20;98(2):e0176423. doi: 10.1128/jvi.01764-23 PMID: 37986840 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

