Implementation of a data control framework to ensure confidentiality, integrity, and availability of high-quality real-world data (RWD) in the NeuroTransData (NTD) registry
- PMID: 35571355
- PMCID: PMC9097675
- DOI: 10.1093/jamiaopen/ooac017
Implementation of a data control framework to ensure confidentiality, integrity, and availability of high-quality real-world data (RWD) in the NeuroTransData (NTD) registry
Abstract
Objective: To implement a dynamic data management and control framework that meets the multiple demands of high data quality, rigorous information technology security, and flexibility to continuously incorporate new methodology for a large disease registry.
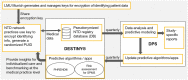
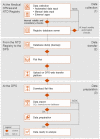
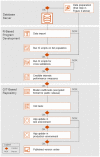
Materials and methods: Guided by relevant sections of the COBIT framework and ISO 27001 standard, we created a data control framework supporting high-quality real-world data (RWD) studies in multiple disease areas. We first mapped and described the entire data journey and identified potential risks for data loss or inconsistencies. Based on this map, we implemented a control framework adhering to best practices and tested its effectiveness through an analysis of random data samples. An internal strategy board was set up to regularly identify and implement potential improvements.
Results: We herein describe the implementation of a data management and control framework for multiple sclerosis, one disease area in the NeuroTransData (NTD) registry that exemplifies the dynamic needs for high-quality RWD analysis. Regular manual and automated analysis of random data samples at multiple checkpoints guided the development and implementation of the framework and continue to ensure timely identification of potential threats to data accuracy.
Discussion and conclusions: High-quality RWD, especially those derived from long-term disease registries, are of increasing importance from regulatory and reimbursement perspectives, requiring owners to provide data of comparable quality to clinical trials. The framework presented herein responds to the call for transparency in real-world analyses and allows doctors and patients to experience an immediate benefit of the collected data for individualized optimal care.
Keywords: data accuracy; information technology; reference standards; registries; trust.
© The Author(s) 2022. Published by Oxford University Press on behalf of the American Medical Informatics Association.
Figures
Similar articles
-
Use of Clinical Data Interchange Standards Consortium (CDISC) Standards for Real-world Data: Expert Perspectives From a Qualitative Delphi Survey.JMIR Med Inform. 2022 Jan 27;10(1):e30363. doi: 10.2196/30363. JMIR Med Inform. 2022. PMID: 35084343 Free PMC article.
-
Preliminary Attainability Assessment of Real-World Data for Answering Major Clinical Research Questions in Breast Cancer Brain Metastasis: Framework Development and Validation Study.J Med Internet Res. 2023 Mar 23;25:e43359. doi: 10.2196/43359. J Med Internet Res. 2023. PMID: 36951923 Free PMC article.
-
RWD-Cockpit: Application for Quality Assessment of Real-world Data.JMIR Form Res. 2022 Oct 18;6(10):e29920. doi: 10.2196/29920. JMIR Form Res. 2022. PMID: 35266872 Free PMC article.
-
Best practices in the real-world data life cycle.PLOS Digit Health. 2022 Jan 18;1(1):e0000003. doi: 10.1371/journal.pdig.0000003. eCollection 2022 Jan. PLOS Digit Health. 2022. PMID: 36812509 Free PMC article. Review.
-
The Value of Real-World Data in Understanding Prostate Cancer Risk and Improving Clinical Care: Examples from Swedish Registries.Cancers (Basel). 2021 Feb 19;13(4):875. doi: 10.3390/cancers13040875. Cancers (Basel). 2021. PMID: 33669624 Free PMC article. Review.
Cited by
-
The critical issue linking lipids and inflammation: Clinical utility of stopping oxidative stress.Front Cardiovasc Med. 2022 Nov 9;9:1042729. doi: 10.3389/fcvm.2022.1042729. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 36439997 Free PMC article. Review.
-
The natural history of primary progressive multiple sclerosis: insights from the German NeuroTransData registry.BMC Neurol. 2023 Jul 5;23(1):258. doi: 10.1186/s12883-023-03273-9. BMC Neurol. 2023. PMID: 37407914 Free PMC article.
-
Cost and Quality of Life of Disability Progression in Multiple Sclerosis Beyond EDSS: Impact of Cognition, Fatigue, and Limb Impairment.Pharmacoecon Open. 2024 Sep;8(5):665-678. doi: 10.1007/s41669-024-00501-x. Epub 2024 Jul 1. Pharmacoecon Open. 2024. PMID: 38949748 Free PMC article.
-
Five-year efficacy outcomes of ocrelizumab in relapsing multiple sclerosis: A propensity-matched comparison of the OPERA studies with other disease-modifying therapies in real-world lines of treatments.J Cent Nerv Syst Dis. 2024 Sep 13;16:11795735241260563. doi: 10.1177/11795735241260563. eCollection 2024. J Cent Nerv Syst Dis. 2024. PMID: 39290861 Free PMC article.
-
Clinical Source Data Production and Quality Control in Real-world Studies: Proposal for Development of the eSource Record System.JMIR Res Protoc. 2022 Dec 23;11(12):e42754. doi: 10.2196/42754. JMIR Res Protoc. 2022. PMID: 36563036 Free PMC article.
References
-
- Cline Amin S. Can Real-World Evidence Transform Healthcare? Recent FDA Activities Indicate Yes. https://www.clinicalleader.com/doc/can-real-world-evidence-transform-hea.... Accessed December 17, 2021.
-
- Graff J. What the Rise of Real-World Evidence Means for the Pharmaceutical Industry: A Closer Look. ISPOR | International Society For Pharmacoeconomics and Outcomes Research. https://www.ispor.org/publications/journals/value-outcomes-spotlight/vos.... Accessed December 17, 2021.
-
- Considerations for the Use of Real-World Data and Real-World Evidence to Support Regulatory Decision-Making for Drug and Biological Products; Draft Guidance for Industry. Federal Register. 2021. https://www.federalregister.gov/documents/2021/12/09/2021-26640/consider.... Accessed December 17, 2021.
LinkOut - more resources
Full Text Sources
Miscellaneous
