Clinical developments of antitumor polymer therapeutics
- PMID: 35528643
- PMCID: PMC9069890
- DOI: 10.1039/c9ra04358f
Clinical developments of antitumor polymer therapeutics
Abstract
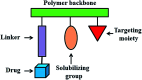
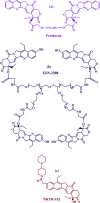
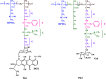
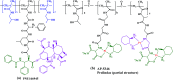
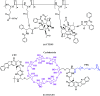
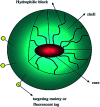
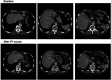
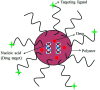
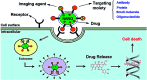
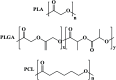
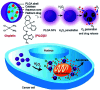
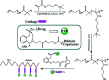
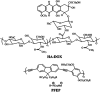
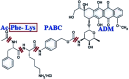
Polymer therapeutics encompasses polymer-drug conjugates that are nano-sized, multicomponent constructs already in the clinic as antitumor compounds, either as single agents or in combination with other organic drug scaffolds. Nanoparticle-based polymer-conjugated therapeutics are poised to become a leading delivery strategy for cancer treatments as they exhibit prolonged half-life, higher stability and selectivity, water solubility, longer clearance time, lower immunogenicity and antigenicity and often also specific targeting to tissues or cells. Compared to free drugs, polymer-tethered drugs preferentially accumulate in the tumor sites unlike conventional chemotherapy which does not discriminate between the cancer cells and healthy cells, thereby causing severe side-effects. It is also desirable that the drug reaches its site of action at a particular concentration and the therapeutic dose remains constant over a sufficiently long period of time. This can be achieved by opting for new formulations possessing polymeric systems of drug carriers. However, many challenges still remain unanswered in polymeric drug conjugates which need to be readdressed and therefore, can broaden the scope of this field. This review highlights some of the antitumor polymer therapeutics including polymer-drug conjugates, polymeric micelles, polymeric liposomes and other polymeric nanoparticles that are currently under investigation.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare that they have no conflicts of interest to disclose.
Figures






Similar articles
-
Advanced targeted therapies in cancer: Drug nanocarriers, the future of chemotherapy.Eur J Pharm Biopharm. 2015 Jun;93:52-79. doi: 10.1016/j.ejpb.2015.03.018. Epub 2015 Mar 23. Eur J Pharm Biopharm. 2015. PMID: 25813885 Review.
-
Polymeric nanoparticles for targeted treatment in oncology: current insights.Int J Nanomedicine. 2015 Feb 2;10:1001-18. doi: 10.2147/IJN.S56932. eCollection 2015. Int J Nanomedicine. 2015. PMID: 25678788 Free PMC article. Review.
-
Cancer-targeted polymeric drugs.Curr Cancer Drug Targets. 2002 Sep;2(3):209-26. doi: 10.2174/1568009023333836. Curr Cancer Drug Targets. 2002. PMID: 12188908 Review.
-
Assessing the pharmacokinetics and toxicology of polymeric micelle conjugated therapeutics.Expert Opin Drug Metab Toxicol. 2021 Mar;17(3):323-332. doi: 10.1080/17425255.2021.1862085. Epub 2020 Dec 21. Expert Opin Drug Metab Toxicol. 2021. PMID: 33292023 Review.
-
Polymer-drug conjugates: current status and future trends.Front Biosci. 2008 Jan 1;13:2744-56. doi: 10.2741/2882. Front Biosci. 2008. PMID: 17981750 Review.
Cited by
-
Non-ionic small amphiphile based nanostructures for biomedical applications.RSC Adv. 2020 Nov 19;10(69):42098-42115. doi: 10.1039/d0ra08092f. eCollection 2020 Nov 17. RSC Adv. 2020. PMID: 35516774 Free PMC article. Review.
-
Recent Advances in the Application of ATRP in the Synthesis of Drug Delivery Systems.Polymers (Basel). 2023 Feb 28;15(5):1234. doi: 10.3390/polym15051234. Polymers (Basel). 2023. PMID: 36904474 Free PMC article. Review.
-
Bioactive Tryptophan-Based Copper Complex with Auxiliary β-Carboline Spectacle Potential on Human Breast Cancer Cells: In Vitro and In Vivo Studies.Molecules. 2021 Mar 14;26(6):1606. doi: 10.3390/molecules26061606. Molecules. 2021. PMID: 33799355 Free PMC article.
-
Poly(methacrylate citric acid) as a Dual Functional Carrier for Tumor Therapy.Pharmaceutics. 2022 Aug 24;14(9):1765. doi: 10.3390/pharmaceutics14091765. Pharmaceutics. 2022. PMID: 36145512 Free PMC article.
-
Recent advances in drug delivery and targeting for the treatment of pancreatic cancer.J Control Release. 2024 Feb;366:231-260. doi: 10.1016/j.jconrel.2023.12.053. Epub 2024 Jan 4. J Control Release. 2024. PMID: 38171473 Review.
References
-
- Rekers N. H. Troost E. G. C. Zegers C. M. L. Germeraad W. T. V. Dubois L. J. Lambin P. Cancer Radiother. 2011;18(5–6):391. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

