Intraocular Pressure-Lowering Efficacy of a Sustained-Release Bimatoprost Implant in Dog Eyes Pretreated with Selective Laser Trabeculoplasty
- PMID: 35442770
- PMCID: PMC9125576
- DOI: 10.1089/jop.2021.0104
Intraocular Pressure-Lowering Efficacy of a Sustained-Release Bimatoprost Implant in Dog Eyes Pretreated with Selective Laser Trabeculoplasty
Abstract
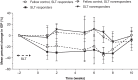
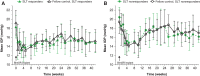
Purpose: To assess the intraocular pressure (IOP)-lowering effect of a biodegradable bimatoprost implant following selective laser trabeculoplasty (SLT) in a canine model. Methods: Unilateral SLT was performed in 11 normotensive, treatment-naive beagle dogs. IOP was measured at baseline (pre-SLT) and weekly post-SLT (≤10 weeks). After IOP returned to baseline or at 10 weeks (whichever occurred first), a sustained-release bimatoprost implant was administered bilaterally in the anterior chamber of each animal. IOP was measured weekly for 4 weeks and then every 2 weeks up to week 42. Results: The main outcomes included the IOP change (%) from baseline, calculated in both eyes in the overall population, SLT responder subgroup (defined by peak IOP reduction from baseline ≥3 mmHg or ≥15% for >1 week post-SLT), and SLT nonresponder subgroup (defined by peak IOP reduction from baseline <3 mmHg or <15%). The bimatoprost implant lowered IOP similarly in both the SLT-treated and fellow SLT-naive eyes. Following bimatoprost implant administration, the mean (standard deviation [SD]) peak IOP reduction from baseline was 34.4% (8.5%) in SLT-treated eyes and 35.7% (5.9%) in fellow SLT-naive eyes. The bimatoprost implant lowered IOP comparably (P > 0.17) in eyes that responded to SLT (mean [SD] peak IOP reduction, 34.6% [10.7%]; n = 6) and those that did not (mean [SD] peak IOP reduction, 34.1% [6.1%]; n = 5). Conclusion: The bimatoprost implant effectively lowered IOP in eyes pretreated with SLT, regardless of response to SLT. The current data suggest that eyes previously treated with SLT can still benefit from the intracameral bimatoprost implant.
Keywords: bimatoprost; glaucoma; intraocular pressure; laser trabeculoplasty; ocular hypertension; sustained-release implant.
Conflict of interest statement
Financial arrangements of the authors with companies whose products may be related to the present report follow, as declared by the authors. C.G., L.R., S.U., S.M., W.O., M.L.G., M.R.R., M.E., and M.D. are employees of AbbVie, Inc., and may hold AbbVie stock. Neither honoraria nor payments were made for authorship.
Figures

Similar articles
-
Short-Term Outcomes of Bimatoprost Sustained-Release Intracameral Implant in Glaucoma.J Glaucoma. 2023 Sep 1;32(9):738-743. doi: 10.1097/IJG.0000000000002271. Epub 2023 Jul 20. J Glaucoma. 2023. PMID: 37523637
-
Phase 3, Randomized Study Comparing Intracameral Bimatoprost Implant 15 µg and Selective Laser Trabeculectomy in Patients with Open-Angle Glaucoma or Ocular Hypertension.Clin Ophthalmol. 2023 Oct 12;17:3023-3036. doi: 10.2147/OPTH.S427976. eCollection 2023. Clin Ophthalmol. 2023. PMID: 37850049 Free PMC article. Clinical Trial.
-
Primary Selective Laser Trabeculoplasty for Open-Angle Glaucoma and Ocular Hypertension: Clinical Outcomes, Predictors of Success, and Safety from the Laser in Glaucoma and Ocular Hypertension Trial.Ophthalmology. 2019 Sep;126(9):1238-1248. doi: 10.1016/j.ophtha.2019.04.012. Epub 2019 Apr 25. Ophthalmology. 2019. PMID: 31028768 Clinical Trial.
-
Selective laser trabeculoplasty versus micropulse laser trabeculoplasty for intraocular pressure control in patients with primary open angle glaucoma: a 12-month retrospective comparative study.Lasers Med Sci. 2023 Apr 17;38(1):102. doi: 10.1007/s10103-023-03771-9. Lasers Med Sci. 2023. PMID: 37067669 Review.
-
Selective Laser Trabeculoplasty: An Update.Asia Pac J Ophthalmol (Phila). 2016 Jan-Feb;5(1):63-9. doi: 10.1097/APO.0000000000000175. Asia Pac J Ophthalmol (Phila). 2016. PMID: 26886122 Review.
Cited by
-
Breaking Barriers in Eye Treatment: Polymeric Nano-Based Drug-Delivery System for Anterior Segment Diseases and Glaucoma.Polymers (Basel). 2023 Mar 9;15(6):1373. doi: 10.3390/polym15061373. Polymers (Basel). 2023. PMID: 36987154 Free PMC article. Review.
-
Using a Novel, Subconjunctival, Sustained-Release Mitomycin C Formulation in a Rabbit Model of Filtration Surgery with Gel Stent Implantation.J Ocul Pharmacol Ther. 2024 Jun;40(5):297-308. doi: 10.1089/jop.2023.0100. Epub 2024 May 2. J Ocul Pharmacol Ther. 2024. PMID: 38687355
-
From Eye Care to Hair Growth: Bimatoprost.Pharmaceuticals (Basel). 2024 Apr 27;17(5):561. doi: 10.3390/ph17050561. Pharmaceuticals (Basel). 2024. PMID: 38794131 Free PMC article. Review.
-
Glaucoma Animal Models beyond Chronic IOP Increase.Int J Mol Sci. 2024 Jan 11;25(2):906. doi: 10.3390/ijms25020906. Int J Mol Sci. 2024. PMID: 38255979 Free PMC article. Review.
References
-
- American Academy of Ophthalmology. Preferred Practice Patterns—Primary open-angle glaucoma. 2020. https://www.aaojournal.org/article/S0161-6420(15)01276-2/pdf Accessed March 16, 2022. - PubMed
-
- European Glaucoma Society Terminology and Guidelines for Glaucoma, 5th Edition. Br. J. Ophthalmol. 105:1–169, 2021. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

