Identification of a novel mechanism for reversal of doxorubicin-induced chemotherapy resistance by TXNIP in triple-negative breast cancer via promoting reactive oxygen-mediated DNA damage
- PMID: 35414060
- PMCID: PMC9005717
- DOI: 10.1038/s41419-022-04783-z
Identification of a novel mechanism for reversal of doxorubicin-induced chemotherapy resistance by TXNIP in triple-negative breast cancer via promoting reactive oxygen-mediated DNA damage
Abstract
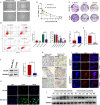
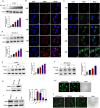
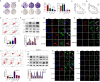
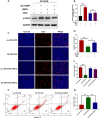
Given that triple-negative breast cancer (TNBC) lacks specific receptors (estrogen and progesterone receptors and human epidermal growth factor receptor 2) and cannot be treated with endocrine therapy, chemotherapy has remained the mainstay of treatment. Drug resistance is reportedly the main obstacle to the clinical use of doxorubicin (DOX) in this patient population. Accordingly, screening molecules related to chemoresistance and studying their specific mechanisms has clinical significance for improving the efficacy of chemotherapy in TNBC patients. Thioredoxin-interacting protein (TXNIP) is a metabolism-related protein that plays a tumor suppressor role in various malignant tumors; however, the specific role of TXNIP in tumor chemoresistance has not been reported. In the present study, we explored the potential molecular mechanism of TXNIP in the chemoresistance of TNBC for the first time. The results showed that TXNIP inhibited the proliferation of TNBC drug-resistant cells and promoted apoptosis in vitro and in vivo. Furthermore, TXNIP promoted the synthesis of reactive oxygen species (ROS) and the accumulation of DNA damage caused by DOX and increased γ-H2AX levels in a time and dose-dependent manner. Moreover, ROS scavenger pretreatment could block DNA damage induced by TXNIP and restore the resistance of TNBC resistant cells to DOX to a certain extent. In addition, we found that the small molecule c-Myc inhibitor 10058-F4 promoted TXNIP expression, increased ROS synthesis in cells, and could enhance the cytotoxicity of chemotherapy drugs in vitro and in vivo when combined with DOX. These results indicated that c-Myc inhibitor 10058-F4 could induce TXNIP upregulation in TNBC drug-resistant cells, and the upregulated TXNIP increased the accumulation of ROS-dependent DNA damage, thereby decreasing chemotherapy resistance of TNBC. Our findings reveal a new mechanism of mediating drug resistance and provide a new drug combination strategy to overcome DOX resistance in TNBC.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
M6A demethylase ALKBH5 regulates FOXO1 mRNA stability and chemoresistance in triple-negative breast cancer.Redox Biol. 2024 Feb;69:102993. doi: 10.1016/j.redox.2023.102993. Epub 2023 Dec 12. Redox Biol. 2024. PMID: 38104484 Free PMC article.
-
Silibinin induces metabolic crisis in triple-negative breast cancer cells by modulating EGFR-MYC-TXNIP axis: potential therapeutic implications.FEBS J. 2021 Jan;288(2):471-485. doi: 10.1111/febs.15353. Epub 2020 May 28. FEBS J. 2021. PMID: 32356386
-
Stat3/Oct-4/c-Myc signal circuit for regulating stemness-mediated doxorubicin resistance of triple-negative breast cancer cells and inhibitory effects of WP1066.Int J Oncol. 2018 Jul;53(1):339-348. doi: 10.3892/ijo.2018.4399. Epub 2018 May 8. Int J Oncol. 2018. PMID: 29750424
-
Molecular targets and therapeutics in chemoresistance of triple-negative breast cancer.Med Oncol. 2021 Nov 23;39(1):14. doi: 10.1007/s12032-021-01610-x. Med Oncol. 2021. PMID: 34812991 Review.
-
Mechanisms of Chemotherapy Resistance in Triple-Negative Breast Cancer-How We Can Rise to the Challenge.Cells. 2019 Aug 22;8(9):957. doi: 10.3390/cells8090957. Cells. 2019. PMID: 31443516 Free PMC article. Review.
Cited by
-
The Cancer Antioxidant Regulation System in Therapeutic Resistance.Antioxidants (Basel). 2024 Jun 27;13(7):778. doi: 10.3390/antiox13070778. Antioxidants (Basel). 2024. PMID: 39061847 Free PMC article. Review.
-
C-Myc Expression in Oral Squamous Cell Carcinoma: Molecular Mechanisms in Cell Survival and Cancer Progression.Pharmaceuticals (Basel). 2022 Jul 19;15(7):890. doi: 10.3390/ph15070890. Pharmaceuticals (Basel). 2022. PMID: 35890188 Free PMC article.
-
Hybrid Nanoparticle-Assisted Chemo-Photothermal Therapy and Photoacoustic Imaging in a Three-Dimensional Breast Cancer Cell Model.Int J Mol Sci. 2023 Dec 12;24(24):17374. doi: 10.3390/ijms242417374. Int J Mol Sci. 2023. PMID: 38139203 Free PMC article.
-
Identification of key ferroptosis genes and mechanisms associated with breast cancer using bioinformatics, machine learning, and experimental validation.Aging (Albany NY). 2024 Jan 19;16(2):1781-1795. doi: 10.18632/aging.205459. Epub 2024 Jan 19. Aging (Albany NY). 2024. PMID: 38244591 Free PMC article.
-
Cpt1c Downregulation Causes Plasma Membrane Remodelling and Anthracycline Resistance in Breast Cancer.Int J Mol Sci. 2023 Jan 4;24(2):946. doi: 10.3390/ijms24020946. Int J Mol Sci. 2023. PMID: 36674468 Free PMC article.
References
-
- Garrido-Castro AC, Lin NU, Polyak K. Insights into molecular classifications of triple-negative breast cancer: improving patient selection for treatment. Cancer Disco. 2019;9:176–98. doi: 10.1158/2159-8290.CD-18-1177. - DOI - PMC - PubMed
-
- Giltnane JM, Balko JM. Rationale for targeting the Ras/MAPK pathway in triple-negative breast cancer. Disco Med. 2014;17:275–83. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

