Acquisition of NOTCH dependence is a hallmark of human intestinal stem cell maturation
- PMID: 35395175
- PMCID: PMC9133587
- DOI: 10.1016/j.stemcr.2022.03.007
Acquisition of NOTCH dependence is a hallmark of human intestinal stem cell maturation
Abstract
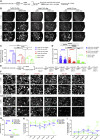
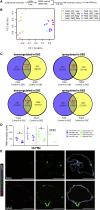
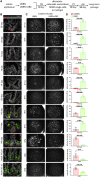
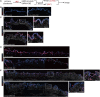
NOTCH signaling is a key regulator involved in maintaining intestinal stem cell (ISC) homeostasis and for balancing differentiation. Using single-cell transcriptomics, we observed that OLFM4, a NOTCH target gene present in ISCs, is first expressed at 13 weeks post-conception in the developing human intestine and increases over time. This led us to hypothesize that the requirement for NOTCH signaling is acquired across human development. To test this, we established a series of epithelium-only organoids (enteroids) from different developmental stages and used γ-secretase inhibitors (dibenzazepine [DBZ] or DAPT) to functionally block NOTCH signaling. Using quantitative enteroid-forming assays, we observed a decrease in enteroid forming efficiency in response to γ-secretase inhibition as development progress. When DBZ was added to cultures and maintained during routine passaging, enteroids isolated from tissue before 20 weeks had higher recovery rates following single-cell serial passaging. Finally, bulk RNA sequencing (RNA-seq) analysis 1 day and 3 days after DBZ treatment showed major differences in the transcriptional changes between developing or adult enteroids. Collectively, these data suggest that ISC dependence on NOTCH signaling increases as the human intestine matures.
Keywords: NOTCH; enteroid; intestinal stem cell; intestine; organoid.
Copyright © 2022 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures



Similar articles
-
The Transcription Co-Repressors MTG8 and MTG16 Regulate Exit of Intestinal Stem Cells From Their Niche and Differentiation Into Enterocyte vs Secretory Lineages.Gastroenterology. 2020 Oct;159(4):1328-1341.e3. doi: 10.1053/j.gastro.2020.06.012. Epub 2020 Jun 15. Gastroenterology. 2020. PMID: 32553763 Free PMC article.
-
ADAM10 regulates Notch function in intestinal stem cells of mice.Gastroenterology. 2014 Oct;147(4):822-834.e13. doi: 10.1053/j.gastro.2014.07.003. Epub 2014 Jul 16. Gastroenterology. 2014. PMID: 25038433 Free PMC article.
-
Development of intestinal organoids as tissue surrogates: cell composition and the epigenetic control of differentiation.Mol Carcinog. 2015 Mar;54(3):189-202. doi: 10.1002/mc.22089. Epub 2013 Sep 21. Mol Carcinog. 2015. PMID: 24115167
-
Notch Signaling in Mammalian Intestinal Stem Cells: Determining Cell Fate and Maintaining Homeostasis.Curr Stem Cell Res Ther. 2019;14(7):583-590. doi: 10.2174/1574888X14666190429143734. Curr Stem Cell Res Ther. 2019. PMID: 31729290 Review.
-
Organoids as a Model System for Studying Notch Signaling in Intestinal Epithelial Homeostasis and Intestinal Cancer.Am J Pathol. 2022 Oct;192(10):1347-1357. doi: 10.1016/j.ajpath.2022.06.008. Epub 2022 Jun 22. Am J Pathol. 2022. PMID: 35752229 Free PMC article. Review.
Cited by
-
Primary human organoids models: Current progress and key milestones.Front Bioeng Biotechnol. 2023 Mar 3;11:1058970. doi: 10.3389/fbioe.2023.1058970. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 36959902 Free PMC article. Review.
-
Activation of fetal-like molecular programs during regeneration in the intestine and beyond.Cell Stem Cell. 2024 Jul 5;31(7):949-960. doi: 10.1016/j.stem.2024.05.009. Cell Stem Cell. 2024. PMID: 38971147 Review.
-
Molecular regulation after mucosal injury and regeneration in ulcerative colitis.Front Mol Biosci. 2022 Oct 13;9:996057. doi: 10.3389/fmolb.2022.996057. eCollection 2022. Front Mol Biosci. 2022. PMID: 36310594 Free PMC article. Review.
-
A matter of differentiation: equine enteroids as a model for the in vivo intestinal epithelium.Vet Res. 2024 Mar 16;55(1):30. doi: 10.1186/s13567-024-01283-0. Vet Res. 2024. PMID: 38493107 Free PMC article.
-
Gut aging: A wane from the normal to repercussion and gerotherapeutic strategies.Heliyon. 2024 Sep 12;10(19):e37883. doi: 10.1016/j.heliyon.2024.e37883. eCollection 2024 Oct 15. Heliyon. 2024. PMID: 39381110 Free PMC article. Review.
References
-
- Barker N., Clevers H. Tracking down the stem cells of the intestine: strategies to identify adult stem cells. Gastroenterology. 2007;133:1755–1760. - PubMed
-
- Barker N., van Es J.H., Kuipers J., Kujala P., van den Born M., Cozijnsen M., Haegebarth A., Korving J., Begthel H., Peters P.J., et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. - PubMed
-
- Beatus P., Lundkvist J., Öberg C., Lendahl U. The Notch 3 intracellular domain represses Notch 1-mediated activation through Hairy/Enhancer of split (HES) promoters. Development. 1999;126:3925–3935. - PubMed
-
- Blondel V.D., Guillaume J.-L., Lambiotte R., Lefebvre E. Fast unfolding of communities in large networks. J. Stat. Mech. Theor. Exp. 2008;2008:1–12.
-
- Bray N.L., Pimentel H., Melsted P., Pachter L. Near-optimal probabilistic RNA-seq quantification. Nat. Biotechnol. 2016;34:525–527. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

