Activation of STING by targeting a pocket in the transmembrane domain
- PMID: 35388221
- PMCID: PMC9098198
- DOI: 10.1038/s41586-022-04559-7
Activation of STING by targeting a pocket in the transmembrane domain
Abstract
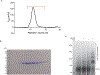
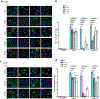
Stimulator of interferon genes (STING) is an adaptor protein in innate immunity against DNA viruses or bacteria1-5. STING-mediated immunity could be exploited in the development of vaccines or cancer immunotherapies. STING is a transmembrane dimeric protein that is located in the endoplasmic reticulum or in the Golgi apparatus. STING is activated by the binding of its cytoplasmic ligand-binding domain to cyclic dinucleotides that are produced by the DNA sensor cyclic GMP-AMP (cGAMP) synthase or by invading bacteria1,6,7. Cyclic dinucleotides induce a conformational change in the STING ligand-binding domain, which leads to a high-order oligomerization of STING that is essential for triggering the downstream signalling pathways8,9. However, the cGAMP-induced STING oligomers tend to dissociate in solution and have not been resolved to high resolution, which limits our understanding of the activation mechanism. Here we show that a small-molecule agonist, compound 53 (C53)10, promotes the oligomerization and activation of human STING through a mechanism orthogonal to that of cGAMP. We determined a cryo-electron microscopy structure of STING bound to both C53 and cGAMP, revealing a stable oligomer that is formed by side-by-side packing and has a curled overall shape. Notably, C53 binds to a cryptic pocket in the STING transmembrane domain, between the two subunits of the STING dimer. This binding triggers outward shifts of transmembrane helices in the dimer, and induces inter-dimer interactions between these helices to mediate the formation of the high-order oligomer. Our functional analyses show that cGAMP and C53 together induce stronger activation of STING than either ligand alone.
© 2022. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Figures

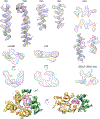


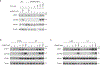




Similar articles
-
Cryo-EM structures of STING reveal its mechanism of activation by cyclic GMP-AMP.Nature. 2019 Mar;567(7748):389-393. doi: 10.1038/s41586-019-0998-5. Epub 2019 Mar 6. Nature. 2019. PMID: 30842659 Free PMC article.
-
Crystal structures of porcine STINGCBD-CDN complexes reveal the mechanism of ligand recognition and discrimination of STING proteins.J Biol Chem. 2019 Jul 26;294(30):11420-11432. doi: 10.1074/jbc.RA119.007367. Epub 2019 Jun 5. J Biol Chem. 2019. PMID: 31167783 Free PMC article.
-
Structural basis of STING binding with and phosphorylation by TBK1.Nature. 2019 Mar;567(7748):394-398. doi: 10.1038/s41586-019-1000-2. Epub 2019 Mar 6. Nature. 2019. PMID: 30842653 Free PMC article.
-
2',3'-Cyclic GMP-AMP Dinucleotides for STING-Mediated Immune Modulation: Principles, Immunotherapeutic Potential, and Synthesis.ChemMedChem. 2022 Jan 19;17(2):e202100671. doi: 10.1002/cmdc.202100671. Epub 2021 Dec 4. ChemMedChem. 2022. PMID: 34807508 Review.
-
Second messenger 2'3'-cyclic GMP-AMP (2'3'-cGAMP): Synthesis, transmission, and degradation.Biochem Pharmacol. 2022 Apr;198:114934. doi: 10.1016/j.bcp.2022.114934. Epub 2022 Jan 31. Biochem Pharmacol. 2022. PMID: 35104477 Review.
Cited by
-
Protein-protein interactions in cGAS-STING pathway: a medicinal chemistry perspective.Future Med Chem. 2024;16(17):1801-1820. doi: 10.1080/17568919.2024.2383164. Epub 2024 Sep 12. Future Med Chem. 2024. PMID: 39263789 Review.
-
The cGAS-STING pathway in diabetic retinopathy and age-related macular degeneration.Future Med Chem. 2023 Apr;15(8):717-729. doi: 10.4155/fmc-2022-0301. Epub 2023 May 11. Future Med Chem. 2023. PMID: 37166075 Free PMC article. Review.
-
STING signaling in the brain: Molecular threats, signaling activities, and therapeutic challenges.Neuron. 2024 Feb 21;112(4):539-557. doi: 10.1016/j.neuron.2023.10.014. Epub 2023 Nov 8. Neuron. 2024. PMID: 37944521 Free PMC article. Review.
-
The activity of disease-causative STING variants can be suppressed by wild-type STING through heterocomplex formation.Front Cell Dev Biol. 2022 Nov 3;10:1037999. doi: 10.3389/fcell.2022.1037999. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36438571 Free PMC article.
-
Crosstalk between macrophages and immunometabolism and their potential roles in tissue repair and regeneration.Heliyon. 2024 Sep 18;10(18):e38018. doi: 10.1016/j.heliyon.2024.e38018. eCollection 2024 Sep 30. Heliyon. 2024. PMID: 39381218 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

