Capsaicin inhibits intestinal Cl- secretion and promotes Na+ absorption by blocking TRPV4 channels in healthy and colitic mice
- PMID: 35314195
- PMCID: PMC9035713
- DOI: 10.1016/j.jbc.2022.101847
Capsaicin inhibits intestinal Cl- secretion and promotes Na+ absorption by blocking TRPV4 channels in healthy and colitic mice
Abstract
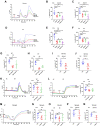
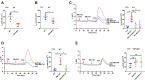
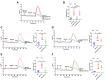
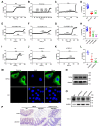
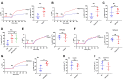
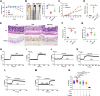
Although capsaicin has been studied extensively as an activator of the transient receptor potential vanilloid cation channel subtype 1 (TRPV1) channels in sensory neurons, little is known about its TRPV1-independent actions in gastrointestinal health and disease. Here, we aimed to investigate the pharmacological actions of capsaicin as a food additive and medication on intestinal ion transporters in mouse models of ulcerative colitis (UC). The short-circuit current (Isc) of the intestine from WT, TRPV1-, and TRPV4-KO mice were measured in Ussing chambers, and Ca2+ imaging was performed on small intestinal epithelial cells. We also performed Western blots, immunohistochemistry, and immunofluorescence on intestinal epithelial cells and on intestinal tissues following UC induction with dextran sodium sulfate. We found that capsaicin did not affect basal intestinal Isc but significantly inhibited carbachol- and caffeine-induced intestinal Isc in WT mice. Capsaicin similarly inhibited the intestinal Isc in TRPV1 KO mice, but this inhibition was absent in TRPV4 KO mice. We also determined that Ca2+ influx via TRPV4 was required for cholinergic signaling-mediated intestinal anion secretion, which was inhibited by capsaicin. Moreover, the glucose-induced jejunal Iscvia Na+/glucose cotransporter was suppressed by TRPV4 activation, which could be relieved by capsaicin. Capsaicin also stimulated ouabain- and amiloride-sensitive colonic Isc. Finally, we found that dietary capsaicin ameliorated the UC phenotype, suppressed hyperaction of TRPV4 channels, and rescued the reduced ouabain- and amiloride-sensitive Isc. We therefore conclude that capsaicin inhibits intestinal Cl- secretion and promotes Na+ absorption predominantly by blocking TRPV4 channels to exert its beneficial anti-colitic action.
Keywords: Na(+)/K(+)-ATPase; TRPV4 channels; epithelial Na(+) channels; short-circuit current; ulcerative colitis.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflicts of interests The authors declare that they have no conflicts of interest with the contents of this article.
Figures

Similar articles
-
Beneficial effect of capsaicin via TRPV4/EDH signals on mesenteric arterioles of normal and colitis mice.J Adv Res. 2022 Jul;39:291-303. doi: 10.1016/j.jare.2021.11.001. Epub 2021 Nov 7. J Adv Res. 2022. PMID: 35777913 Free PMC article.
-
Capsaicin induces NKCC1 internalization and inhibits chloride secretion in colonic epithelial cells independently of TRPV1.Am J Physiol Gastrointest Liver Physiol. 2013 Jan 15;304(2):G142-56. doi: 10.1152/ajpgi.00483.2011. Epub 2012 Nov 8. Am J Physiol Gastrointest Liver Physiol. 2013. PMID: 23139219 Free PMC article.
-
Zinc pyrithione ameliorates colitis in mice by interacting on intestinal epithelial TRPA1 and TRPV4 channels.Life Sci. 2024 Dec 1;358:123090. doi: 10.1016/j.lfs.2024.123090. Epub 2024 Oct 9. Life Sci. 2024. PMID: 39384148
-
Differential effects of TRPV channel block on polymodal activation of rat cutaneous nociceptors in vitro.Exp Brain Res. 2009 Jun;196(1):31-44. doi: 10.1007/s00221-009-1808-3. Epub 2009 Apr 30. Exp Brain Res. 2009. PMID: 19404626 Review.
-
Transient receptor potential vanilloid 1 (TRPV1)-independent actions of capsaicin on cellular excitability and ion transport.Med Res Rev. 2023 Jul;43(4):1038-1067. doi: 10.1002/med.21945. Epub 2023 Mar 14. Med Res Rev. 2023. PMID: 36916676 Review.
Cited by
-
Single-cell RNA sequencing uncovers a neuron-like macrophage subset associated with cancer pain.Sci Adv. 2022 Oct 7;8(40):eabn5535. doi: 10.1126/sciadv.abn5535. Epub 2022 Oct 7. Sci Adv. 2022. PMID: 36206343 Free PMC article.
-
TET1-TRPV4 Signaling Contributes to Bone Cancer Pain in Rats.Brain Sci. 2023 Apr 10;13(4):644. doi: 10.3390/brainsci13040644. Brain Sci. 2023. PMID: 37190609 Free PMC article.
-
Are We Ready to Recommend Capsaicin for Disorders Other Than Neuropathic Pain?Nutrients. 2023 Oct 21;15(20):4469. doi: 10.3390/nu15204469. Nutrients. 2023. PMID: 37892544 Free PMC article. Review.
-
Ulcerative colitis: molecular insights and intervention therapy.Mol Biomed. 2024 Oct 10;5(1):42. doi: 10.1186/s43556-024-00207-w. Mol Biomed. 2024. PMID: 39384730 Free PMC article. Review.
-
Calcitonin gene‑related peptide alleviates hyperoxia‑induced human alveolar cell injury via the CGRPR/TRPV1/Ca2+ axis.Mol Med Rep. 2024 Jul;30(1):110. doi: 10.3892/mmr.2024.13234. Epub 2024 May 2. Mol Med Rep. 2024. PMID: 38695251 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

