Exerkines in health, resilience and disease
- PMID: 35304603
- PMCID: PMC9554896
- DOI: 10.1038/s41574-022-00641-2
Exerkines in health, resilience and disease
Abstract
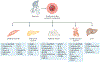
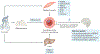
The health benefits of exercise are well-recognized and are observed across multiple organ systems. These beneficial effects enhance overall resilience, healthspan and longevity. The molecular mechanisms that underlie the beneficial effects of exercise, however, remain poorly understood. Since the discovery in 2000 that muscle contraction releases IL-6, the number of exercise-associated signalling molecules that have been identified has multiplied. Exerkines are defined as signalling moieties released in response to acute and/or chronic exercise, which exert their effects through endocrine, paracrine and/or autocrine pathways. A multitude of organs, cells and tissues release these factors, including skeletal muscle (myokines), the heart (cardiokines), liver (hepatokines), white adipose tissue (adipokines), brown adipose tissue (baptokines) and neurons (neurokines). Exerkines have potential roles in improving cardiovascular, metabolic, immune and neurological health. As such, exerkines have potential for the treatment of cardiovascular disease, type 2 diabetes mellitus and obesity, and possibly in the facilitation of healthy ageing. This Review summarizes the importance and current state of exerkine research, prevailing challenges and future directions.
© 2022. Springer Nature Limited.
Conflict of interest statement
Competing interests
The other authors declare no competing interests.
Figures
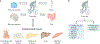

Comment in
-
Reply to 'Lactate as a major myokine and exerkine'.Nat Rev Endocrinol. 2022 Nov;18(11):713. doi: 10.1038/s41574-022-00726-y. Nat Rev Endocrinol. 2022. PMID: 35915255 No abstract available.
-
Lactate as a major myokine and exerkine.Nat Rev Endocrinol. 2022 Nov;18(11):712. doi: 10.1038/s41574-022-00724-0. Nat Rev Endocrinol. 2022. PMID: 35915256 No abstract available.
Similar articles
-
Exploring exercise-driven exerkines: unraveling the regulation of metabolism and inflammation.PeerJ. 2024 Apr 29;12:e17267. doi: 10.7717/peerj.17267. eCollection 2024. PeerJ. 2024. PMID: 38699186 Free PMC article. Review.
-
Exerkines and cardiometabolic benefits of exercise: from bench to clinic.EMBO Mol Med. 2024 Mar;16(3):432-444. doi: 10.1038/s44321-024-00027-z. Epub 2024 Feb 6. EMBO Mol Med. 2024. PMID: 38321233 Free PMC article. Review.
-
Highlighting the idea of exerkines in the management of cancer patients with cachexia: novel insights and a critical review.BMC Cancer. 2023 Sep 20;23(1):889. doi: 10.1186/s12885-023-11391-3. BMC Cancer. 2023. PMID: 37730552 Free PMC article. Review.
-
Metabolic Adaptation in Obesity and Type II Diabetes: Myokines, Adipokines and Hepatokines.Int J Mol Sci. 2016 Dec 22;18(1):8. doi: 10.3390/ijms18010008. Int J Mol Sci. 2016. PMID: 28025491 Free PMC article. Review.
-
Factors mediating exercise-induced organ crosstalk.Acta Physiol (Oxf). 2022 Feb;234(2):e13766. doi: 10.1111/apha.13766. Epub 2022 Jan 18. Acta Physiol (Oxf). 2022. PMID: 34981891 Review.
Cited by
-
Functional assays reflective of cancer hallmarks in BT-549 cells are not impacted by media supplemented with exercise-trained plasma.Exp Physiol. 2024 Jul;109(7):1124-1133. doi: 10.1113/EP091383. Epub 2023 Nov 22. Exp Physiol. 2024. PMID: 37991325 Free PMC article.
-
Irisin: An anti-inflammatory exerkine in aging and redox-mediated comorbidities.Front Endocrinol (Lausanne). 2023 Feb 10;14:1106529. doi: 10.3389/fendo.2023.1106529. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 36843614 Free PMC article. Review.
-
β-cell dynamics in type 2 diabetes and in dietary and exercise interventions.J Mol Cell Biol. 2022 Nov 30;14(7):mjac046. doi: 10.1093/jmcb/mjac046. J Mol Cell Biol. 2022. PMID: 35929791 Free PMC article. Review.
-
Nordic Walking training in BungyPump form improves cognitive functions and physical performance and induces changes in amino acids and kynurenine profiles in older adults.Front Endocrinol (Lausanne). 2023 Sep 11;14:1151184. doi: 10.3389/fendo.2023.1151184. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37766686 Free PMC article.
-
Associations between physical activity levels and ATPase inhibitory factor 1 concentrations in older adults.J Sport Health Sci. 2024 May;13(3):409-418. doi: 10.1016/j.jshs.2023.09.009. Epub 2023 Sep 23. J Sport Health Sci. 2024. PMID: 37748689 Free PMC article.
References
-
- Katzmarzyk PT, Church TS, Craig CL & Bouchard C.Sitting time and mortality from all causes, cardiovascular disease, and cancer. Med. Sci. Sports Exerc 41, 998–1005 (2009). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U24 DK112348/DK/NIDDK NIH HHS/United States
- R01 AG060542/AG/NIA NIH HHS/United States
- P30 DK040561/DK/NIDDK NIH HHS/United States
- R03 AG067960/AG/NIA NIH HHS/United States
- DH_/Department of Health/United Kingdom
- U01 AR071133/AR/NIAMS NIH HHS/United States
- R01 HL138738/HL/NHLBI NIH HHS/United States
- P30 DK020572/DK/NIDDK NIH HHS/United States
- K23 HL150327/HL/NHLBI NIH HHS/United States
- U54 DK102556/DK/NIDDK NIH HHS/United States
- R01 HL142879/HL/NHLBI NIH HHS/United States
- R01 HL165786/HL/NHLBI NIH HHS/United States
- R01 DK098203/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials

