Infection of non-cancer cells: A barrier or support for oncolytic virotherapy?
- PMID: 35284629
- PMCID: PMC8898763
- DOI: 10.1016/j.omto.2022.02.004
Infection of non-cancer cells: A barrier or support for oncolytic virotherapy?
Abstract
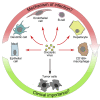
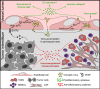
Oncolytic viruses are designed to specifically target cancer cells, sparing normal cells. Although numerous studies demonstrate the ability of oncolytic viruses to infect a wide range of non-tumor cells, the significance of this phenomenon for cancer virotherapy is poorly understood. To fill the gap, we summarize the data on infection of non-cancer targets by oncolytic viruses with a special focus on tumor microenvironment and secondary lymphoid tissues. The review aims to address two major questions: how do attenuated viruses manage to infect normal cells, and whether it is of importance for oncolytic virotherapy.
Keywords: cancer therapy; infection of non-cancer cells; oncolytic virus; secondary lymphoid tissues; tumor microenvironment.
© 2022 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Modeling oncolytic virotherapy: is complete tumor-tropism too much of a good thing?J Theor Biol. 2014 Oct 7;358:166-78. doi: 10.1016/j.jtbi.2014.04.030. Epub 2014 May 5. J Theor Biol. 2014. PMID: 24810840
-
Oncolytic Viruses and Their Potential as a Therapeutic Opportunity in Osteosarcoma.Adv Exp Med Biol. 2020;1258:77-89. doi: 10.1007/978-3-030-43085-6_5. Adv Exp Med Biol. 2020. PMID: 32767235 Review.
-
Employing RNA viruses to fight cancer: novel insights into oncolytic virotherapy.Biol Chem. 2017 Jul 26;398(8):891-909. doi: 10.1515/hsz-2017-0103. Biol Chem. 2017. PMID: 28441138 Review.
-
Oncolytic Viruses for Malignant Glioma: On the Verge of Success?Viruses. 2021 Jul 2;13(7):1294. doi: 10.3390/v13071294. Viruses. 2021. PMID: 34372501 Free PMC article. Review.
-
Stem Cell-Based Cell Carrier for Targeted Oncolytic Virotherapy: Translational Opportunity and Open Questions.Viruses. 2015 Nov 27;7(12):6200-17. doi: 10.3390/v7122921. Viruses. 2015. PMID: 26633462 Free PMC article. Review.
Cited by
-
Immunovirotherapy: The role of antibody based therapeutics combination with oncolytic viruses.Front Immunol. 2022 Oct 13;13:1012806. doi: 10.3389/fimmu.2022.1012806. eCollection 2022. Front Immunol. 2022. PMID: 36311790 Free PMC article. Review.
-
In Vivo Tracking for Oncolytic Adenovirus Interactions with Liver Cells.Biomedicines. 2022 Jul 13;10(7):1697. doi: 10.3390/biomedicines10071697. Biomedicines. 2022. PMID: 35885002 Free PMC article.
-
Erythrocyte-Leveraged Oncolytic Virotherapy (ELeOVt): Oncolytic Virus Assembly on Erythrocyte Surface to Combat Pulmonary Metastasis and Alleviate Side Effects.Adv Sci (Weinh). 2024 Feb;11(5):e2303907. doi: 10.1002/advs.202303907. Epub 2023 Nov 23. Adv Sci (Weinh). 2024. PMID: 37997186 Free PMC article.
-
Engineered Oncolytic Adenoviruses: An Emerging Approach for Cancer Therapy.Pathogens. 2022 Oct 4;11(10):1146. doi: 10.3390/pathogens11101146. Pathogens. 2022. PMID: 36297203 Free PMC article. Review.
-
Developing Oncolytic Viruses for the Treatment of Cervical Cancer.Cells. 2023 Jul 13;12(14):1838. doi: 10.3390/cells12141838. Cells. 2023. PMID: 37508503 Free PMC article. Review.
References
-
- Luker K., Hutchens M., Schultz T., Pekosz A., Luker G. Bioluminescence imaging of vaccinia virus: effects of interferon on viral replication and spread. Virology. 2005;341:284–300. - PubMed
-
- Breitbach C.J., Burke J., Jonker D., Stephenson J., Haas A.R., Chow L.Q.M., Nieva J., Hwang T.-H., Moon A., Patt R., et al. Intravenous delivery of a multi-mechanistic cancer-targeted oncolytic poxvirus in humans. Nature. 2011;477:99–102. 2011 4777362. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

