Mechanism and Disease Association With a Ubiquitin Conjugating E2 Enzyme: UBE2L3
- PMID: 35265070
- PMCID: PMC8899012
- DOI: 10.3389/fimmu.2022.793610
Mechanism and Disease Association With a Ubiquitin Conjugating E2 Enzyme: UBE2L3
Abstract
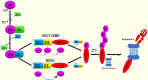
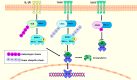
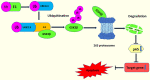
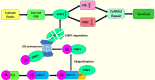
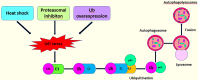
Ubiquitin conjugating enzyme E2 is an important component of the post-translational protein ubiquitination pathway, which mediates the transfer of activated ubiquitin to substrate proteins. UBE2L3, also called UBcH7, is one of many E2 ubiquitin conjugating enzymes that participate in the ubiquitination of many substrate proteins and regulate many signaling pathways, such as the NF-κB, GSK3β/p65, and DSB repair pathways. Studies on UBE2L3 have found that it has an abnormal expression in many diseases, mainly immune diseases, tumors and Parkinson's disease. It can also promote the occurrence and development of these diseases. Resultantly, UBE2L3 may become an important target for some diseases. Herein, we review the structure of UBE2L3, and its mechanism in diseases, as well as diseases related to UBE2L3 and discuss the related challenges.
Keywords: NF-κB; Parkinson’s disease; UBE2L3; Ubiquitination; cancer; immune diseases; p53; p62.
Copyright © 2022 Zhang, Huo, Liu, Su, Zhao and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
UBE2L3 polymorphism amplifies NF-κB activation and promotes plasma cell development, linking linear ubiquitination to multiple autoimmune diseases.Am J Hum Genet. 2015 Feb 5;96(2):221-34. doi: 10.1016/j.ajhg.2014.12.024. Epub 2015 Jan 29. Am J Hum Genet. 2015. PMID: 25640675 Free PMC article.
-
UBE2L3 promotes squamous cell carcinoma progression in the oral cavity and hypopharynx via activating the NF-κB signaling by increasing IκBα degradation.Cell Biol Int. 2022 May;46(5):806-818. doi: 10.1002/cbin.11772. Epub 2022 Mar 7. Cell Biol Int. 2022. PMID: 35128752
-
Stabilization of p27Kip1/CDKN1B by UBCH7/UBE2L3 catalyzed ubiquitinylation: a new paradigm in cell-cycle control.FASEB J. 2019 Jan;33(1):1235-1247. doi: 10.1096/fj.201800960R. Epub 2018 Aug 16. FASEB J. 2019. PMID: 30113882 Free PMC article.
-
The Ubiquitin Conjugating Enzyme: An Important Ubiquitin Transfer Platform in Ubiquitin-Proteasome System.Int J Mol Sci. 2020 Apr 21;21(8):2894. doi: 10.3390/ijms21082894. Int J Mol Sci. 2020. PMID: 32326224 Free PMC article. Review.
-
Mechanism and disease association of E2-conjugating enzymes: lessons from UBE2T and UBE2L3.Biochem J. 2016 Oct 15;473(20):3401-3419. doi: 10.1042/BCJ20160028. Biochem J. 2016. PMID: 27729585 Free PMC article. Review.
Cited by
-
Identification of susceptibility loci and relevant cell type for IgA nephropathy in Han Chinese by integrative genome-wide analysis.Front Med. 2024 Oct;18(5):862-877. doi: 10.1007/s11684-024-1086-2. Epub 2024 Sep 30. Front Med. 2024. PMID: 39343836
-
Investigating the shared genetic basis of inflammatory bowel disease and systemic lupus erythematosus using genetic overlap analysis.BMC Genomics. 2024 Sep 16;25(1):868. doi: 10.1186/s12864-024-10787-0. BMC Genomics. 2024. PMID: 39285290 Free PMC article.
-
Discovery of new myositis genetic associations through leveraging other immune-mediated diseases.HGG Adv. 2024 Oct 10;5(4):100336. doi: 10.1016/j.xhgg.2024.100336. Epub 2024 Jul 22. HGG Adv. 2024. PMID: 39044428 Free PMC article.
-
The membrane-associated ubiquitin ligase MARCHF8 stabilizes the human papillomavirus oncoprotein E7 by degrading CUL1 and UBE2L3 in head and neck cancer.J Virol. 2024 Feb 20;98(2):e0172623. doi: 10.1128/jvi.01726-23. Epub 2024 Jan 16. J Virol. 2024. PMID: 38226814 Free PMC article.
-
The membrane-associated ubiquitin ligase MARCHF8 stabilizes the human papillomavirus oncoprotein E7 by degrading CUL1 and UBE2L3 in head and neck cancer.bioRxiv [Preprint]. 2023 Nov 4:2023.11.03.565564. doi: 10.1101/2023.11.03.565564. bioRxiv. 2023. Update in: J Virol. 2024 Feb 20;98(2):e0172623. doi: 10.1128/jvi.01726-23. PMID: 37961092 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

