Clinical characteristics, surveillance, treatment allocation, and outcomes of non-alcoholic fatty liver disease-related hepatocellular carcinoma: a systematic review and meta-analysis
- PMID: 35255263
- PMCID: PMC9718369
- DOI: 10.1016/S1470-2045(22)00078-X
Clinical characteristics, surveillance, treatment allocation, and outcomes of non-alcoholic fatty liver disease-related hepatocellular carcinoma: a systematic review and meta-analysis
Abstract
Background: The clinical presentation and outcomes of non-alcoholic fatty liver disease (NAFLD)-related hepatocellular carcinoma are unclear when compared with hepatocellular carcinoma due to other causes. We aimed to establish the prevalence, clinical features, surveillance rates, treatment allocation, and outcomes of NAFLD-related hepatocellular carcinoma.
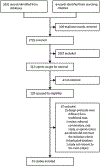
Methods: In this systematic review and meta-analysis, we searched MEDLINE and Embase from inception until Jan 17, 2022, for articles in English that compared clinical features, and outcomes of NAFLD-related hepatocellular carcinoma versus hepatocellular carcinoma due to other causes. We included cross-sectional and longitudinal observational studies and excluded paediatric studies. Study-level data were extracted from the published reports. The primary outcomes were (1) the proportion of hepatocellular carcinoma secondary to NAFLD, (2) comparison of patient and tumour characteristics of NAFLD-related hepatocellular carcinoma versus other causes, and (3) comparison of surveillance, treatment allocation, and overall and disease-free survival outcomes of NAFLD-related versus non-NAFLD-related hepatocellular carcinoma. We analysed proportional data using a generalised linear mixed model. Pairwise meta-analysis was done to obtain odds ratio (OR) or mean difference, comparing NAFLD-related with non-NAFLD-related hepatocellular carcinoma. We evaluated survival outcomes using pooled analysis of hazard ratios.
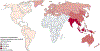
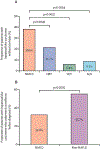
Findings: Of 3631 records identified, 61 studies (done between January, 1980, and May, 2021; 94 636 patients) met inclusion criteria. Overall, the proportion of hepatocellular carcinoma cases secondary to NAFLD was 15·1% (95% CI 11·9-18·9). Patients with NAFLD-related hepatocellular carcinoma were older (p<0·0001), had higher BMI (p<0·0001), and were more likely to present with metabolic comorbidities (diabetes [p<0·0001], hypertension [p<0·0001], and hyperlipidaemia [p<0·0001]) or cardiovascular disease at presentation (p=0·0055) than patients with hepatocellular carcinoma due to other causes. They were also more likely to be non-cirrhotic (38·5%, 27·9-50·2 vs 14·6%, 8·7-23·4 for hepatocellular carcinoma due to other causes; p<0·0001). Patients with NAFLD-related hepatocellular carcinoma had larger tumour diameters (p=0·0087), were more likely to have uninodular lesions (p=0·0003), and had similar odds of Barcelona Clinic Liver Cancer stages, TNM stages, alpha fetoprotein concentration, and Eastern Cooperative Oncology Group (ECOG) performance status to patients with non-NAFLD-related hepatocellular carcinoma. A lower proportion of patients with NAFLD-related hepatocellular carcinoma underwent surveillance (32·8%, 12·0-63·7) than did patients with hepatocellular carcinoma due to other causes (55·7%, 24·0-83·3; p<0·0001). There were no significant differences in treatment allocation (curative therapy, palliative therapy, and best supportive care) between patients with NAFLD-related hepatocellular carcinoma and those with hepatocellular carcinoma due to other causes. Overall survival did not differ between the two groups (hazard ratio 1·05, 95% CI 0·92-1·20, p=0·43), but disease-free survival was longer for patients with NAFLD-related hepatocellular carcinoma (0·79, 0·63-0·99; p=0·044). There was substantial heterogeneity in most analyses (I2>75%), and all articles had low-to-moderate risk of bias.
Interpretation: NAFLD-related hepatocellular carcinoma is associated with a higher proportion of patients without cirrhosis and lower surveillance rates than hepatocellular carcinoma due to other causes. Surveillance strategies should be developed for patients with NAFLD without cirrhosis who are at high risk of developing hepatocellular carcinoma.
Funding: None.
Copyright © 2022 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests AJS is President of Sanyal Biotechnology and has stock options in Genfit, Akarna, Tiziana, Indalo, Durect, and Galmed; has served as a consultant to AstraZeneca, Nitto Denko, Enyo, Ardelyx, Conatus, Nimbus, Amarin, Salix, Tobira, Takeda, Jannsen, Gilead, Terns, Birdrock, Merck, Valeant, Boehringer-Ingelheim, Lilly, Hemoshear, Zafgen, Novartis, Novo Nordisk, Pfizer, Exhalenz, and Genfit; and has been an unpaid consultant to Intercept, Echosens, Immuron, Galectin, Fractyl, Syntlogic, Affimune, Chemomab, Zydus, Nordic Bioscience, Albireo, Prosciento, Surrozen, and Bristol Myers Squibb. His institution has received grant support from Gilead, Salix, Tobira, Bristol Myers Squibb, Shire, Intercept, Merck, AstraZeneca, Malinckrodt, Cumberland, and Novartis. He receives royalties from Elsevier and UptoDate. RL serves as a consultant to Aardvark Therapeutics, Altimmune, Anylam/Regeneron, Amgen, Arrowhead Pharmaceuticals, AstraZeneca, Bristol Myers Squibb, CohBar, Eli Lilly, Galmed, Gilead, Glympse bio, Hightide, Inipharma, Intercept, Inventiva, Ionis, Janssen, Madrigal, Metacrine, NGM Biopharmaceuticals, Novartis, Novo Nordisk, Merck, Pfizer, Sagimet, Theratechnologies, 89 bio, Terns Pharmaceuticals, and Viking Therapeutics. His institutions received research grants from Arrowhead Pharmaceuticals, AstraZeneca, Boehringer-Ingelheim, Bristol Myers Squibb, Eli Lilly, Galectin Therapeutics, Galmed Pharmaceuticals, Gilead, Intercept, Hanmi, Intercept, Inventiva, Ionis, Janssen, Madrigal Pharmaceuticals, Merck, NGM Biopharmaceuticals, Novo Nordisk, Merck, Pfizer, Sonic Incytes and Terns Pharmaceuticals. RL is also the co-founder of LipoNexus. DQH has served as an advisory board member for Eisai. All other authors declare no competing interests.
Figures
Comment in
-
Clinical features and outcomes of NAFLD-related hepatocellular carcinoma.Lancet Oncol. 2022 Jun;23(6):e243. doi: 10.1016/S1470-2045(22)00272-8. Lancet Oncol. 2022. PMID: 35654060 No abstract available.
-
Clinical features and outcomes of NAFLD-related hepatocellular carcinoma - Authors' reply.Lancet Oncol. 2022 Jun;23(6):e244. doi: 10.1016/S1470-2045(22)00265-0. Lancet Oncol. 2022. PMID: 35654061 No abstract available.
Similar articles
-
Survival after treatment with curative intent for hepatocellular carcinoma among patients with vs without non-alcoholic fatty liver disease.Aliment Pharmacol Ther. 2017 Dec;46(11-12):1061-1069. doi: 10.1111/apt.14342. Epub 2017 Sep 28. Aliment Pharmacol Ther. 2017. PMID: 28960360
-
Temporal trends, clinical patterns and outcomes of NAFLD-related HCC in patients undergoing liver resection over a 20-year period.Aliment Pharmacol Ther. 2017 Nov;46(9):856-863. doi: 10.1111/apt.14261. Epub 2017 Aug 31. Aliment Pharmacol Ther. 2017. PMID: 28857208
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
Hepatocellular carcinoma in patients with nonalcoholic fatty liver disease: A systematic review and meta-analysis: HCC and Steatosis or Steatohepatitis.Neoplasia. 2022 Aug;30:100809. doi: 10.1016/j.neo.2022.100809. Epub 2022 May 27. Neoplasia. 2022. PMID: 35636146 Free PMC article. Review.
-
Distinctive features of hepatocellular carcinoma in non-alcoholic fatty liver disease.Lancet Gastroenterol Hepatol. 2016 Oct;1(2):156-164. doi: 10.1016/S2468-1253(16)30018-8. Epub 2016 Sep 8. Lancet Gastroenterol Hepatol. 2016. PMID: 28404072 Review.
Cited by
-
NAFLD and NAFLD-related HCC in Asia: Burden and Surveillance.J Clin Exp Hepatol. 2024 Jan-Feb;14(1):101213. doi: 10.1016/j.jceh.2023.06.013. Epub 2023 Jul 5. J Clin Exp Hepatol. 2024. PMID: 38076360 Free PMC article. Review.
-
Diagnostic Performance of Abbreviated MRI for HCC Detection in Patients with Non-alcoholic Fatty Liver Disease.J Clin Exp Hepatol. 2024 Jan-Feb;14(1):101276. doi: 10.1016/j.jceh.2023.08.012. Epub 2023 Aug 29. J Clin Exp Hepatol. 2024. PMID: 38076364 Free PMC article.
-
NASH and Hepatocellular Carcinoma: Immunology and Immunotherapy.Clin Cancer Res. 2023 Feb 1;29(3):513-520. doi: 10.1158/1078-0432.CCR-21-1258. Clin Cancer Res. 2023. PMID: 36166660 Free PMC article. Review.
-
Time course of western diet (WD) induced nonalcoholic steatohepatitis (NASH) in female and male Ldlr-/- mice.PLoS One. 2023 Oct 11;18(10):e0292432. doi: 10.1371/journal.pone.0292432. eCollection 2023. PLoS One. 2023. PMID: 37819925 Free PMC article.
-
Banting memorial lecture 2022: 'Type 2 diabetes and nonalcoholic fatty liver disease: Partners in crime'.Diabet Med. 2022 Oct;39(10):e14912. doi: 10.1111/dme.14912. Epub 2022 Jul 20. Diabet Med. 2022. PMID: 35790023 Free PMC article. Review.
References
-
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021; 71: 209–49. - PubMed
-
- Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Primers 2021; 7: 6. - PubMed
-
- Muthiah MD, Sanyal AJ. Burden of disease due to nonalcoholic fatty liver disease. Gastroenterol Clin North Am 2020; 49: 1–23. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

