Tuft Cells and Their Role in Intestinal Diseases
- PMID: 35237268
- PMCID: PMC8884241
- DOI: 10.3389/fimmu.2022.822867
Tuft Cells and Their Role in Intestinal Diseases
Abstract
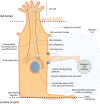
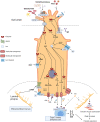
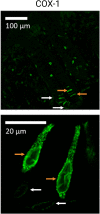
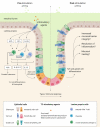
The interests in intestinal epithelial tuft cells, their basic physiology, involvement in immune responses and relevance for gut diseases, have increased dramatically over the last fifteen years. A key discovery in 2016 of their close connection to helminthic and protozoan infection has further spurred the exploration of these rare chemosensory epithelial cells. Although very sparse in number, tuft cells are now known as important sentinels in the gastrointestinal tract as they monitor intestinal content using succinate as well as sweet and bitter taste receptors. Upon stimulation, tuft cells secrete a broad palette of effector molecules, including interleukin-25, prostaglandin E2 and D2, cysteinyl leukotriene C4, acetylcholine, thymic stromal lymphopoietin, and β-endorphins, some of which with immunomodulatory functions. Tuft cells have proven indispensable in anti-helminthic and anti-protozoan immunity. Most studies on tuft cells are based on murine experiments using double cortin-like kinase 1 (DCLK1) as a marker, while human intestinal tuft cells can be identified by their expression of the cyclooxygenase-1 enzyme. So far, only few studies have examined tuft cells in humans and their relation to gut disease. Here, we present an updated view on intestinal epithelial tuft cells, their physiology, immunological hub function, and their involvement in human disease. We close with a discussion on how tuft cells may have potential therapeutic value in a clinical context.
Keywords: Crohn’s disease; chemosensing; colorectal neoplasia; inflammation; inflammatory bowel disease; intestine; tuft cells; ulcerative colitis.
Copyright © 2022 Hendel, Kellermann, Hausmann, Bindslev, Jensen and Nielsen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
[Characteristics of intestinal tuft cells and their role in the pathomechanism of inflammatory bowel disease and colorectal carcinoma].Orv Hetil. 2023 Nov 5;164(44):1727-1735. doi: 10.1556/650.2023.32898. Print 2023 Nov 5. Orv Hetil. 2023. PMID: 37930381 Hungarian.
-
Dclk1 in tuft cells promotes inflammation-driven epithelial restitution and mitigates chronic colitis.Cell Death Differ. 2019 Sep;26(9):1656-1669. doi: 10.1038/s41418-018-0237-x. Epub 2018 Nov 26. Cell Death Differ. 2019. PMID: 30478383 Free PMC article.
-
MicroRNA-195 regulates Tuft cell function in the intestinal epithelium by altering translation of DCLK1.Am J Physiol Cell Physiol. 2021 Jun 1;320(6):C1042-C1054. doi: 10.1152/ajpcell.00597.2020. Epub 2021 Mar 31. Am J Physiol Cell Physiol. 2021. PMID: 33788631 Free PMC article.
-
Dclk1-expressing tuft cells: critical modulators of the intestinal niche?Am J Physiol Gastrointest Liver Physiol. 2017 Oct 1;313(4):G285-G299. doi: 10.1152/ajpgi.00073.2017. Epub 2017 Jul 6. Am J Physiol Gastrointest Liver Physiol. 2017. PMID: 28684459 Free PMC article. Review.
-
Regulation of immune responses by tuft cells.Nat Rev Immunol. 2019 Sep;19(9):584-593. doi: 10.1038/s41577-019-0176-x. Nat Rev Immunol. 2019. PMID: 31114038 Free PMC article. Review.
Cited by
-
Gastrointestinal Tract Homeostasis: The Role of the Inositol Polyphosphate Multikinase.Cell Mol Gastroenterol Hepatol. 2022;14(6):1332-1333. doi: 10.1016/j.jcmgh.2022.09.001. Epub 2022 Sep 23. Cell Mol Gastroenterol Hepatol. 2022. PMID: 36162437 Free PMC article. No abstract available.
-
Capsaicin: Emerging Pharmacological and Therapeutic Insights.Curr Issues Mol Biol. 2024 Jul 24;46(8):7895-7943. doi: 10.3390/cimb46080468. Curr Issues Mol Biol. 2024. PMID: 39194685 Free PMC article. Review.
-
p53 suppresses MHC class II presentation by intestinal epithelium to protect against radiation-induced gastrointestinal syndrome.Nat Commun. 2024 Jan 2;15(1):137. doi: 10.1038/s41467-023-44390-w. Nat Commun. 2024. PMID: 38167344 Free PMC article.
-
BMP signaling in the intestinal epithelium drives a critical feedback loop to restrain IL-13-driven tuft cell hyperplasia.Sci Immunol. 2022 May 13;7(71):eabl6543. doi: 10.1126/sciimmunol.abl6543. Epub 2022 May 13. Sci Immunol. 2022. PMID: 35559665 Free PMC article.
-
Vaccine Strategies to Elicit Mucosal Immunity.Vaccines (Basel). 2024 Feb 13;12(2):191. doi: 10.3390/vaccines12020191. Vaccines (Basel). 2024. PMID: 38400174 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

