Inhibition of TRPC3 channels by a novel pyrazole compound confers antiseizure effects
- PMID: 35179226
- PMCID: PMC9007831
- DOI: 10.1111/epi.17190
Inhibition of TRPC3 channels by a novel pyrazole compound confers antiseizure effects
Abstract
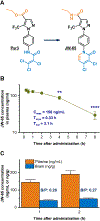
Objective: As a key member of the transient receptor potential (TRP) superfamily, TRP canonical 3 (TRPC3) regulates calcium homeostasis and contributes to neuronal excitability. Ablation of TRPC3 lessens pilocarpine-induced seizures in mice, suggesting that TRPC3 inhibition might represent a novel antiseizure strategy. Among current TRPC3 inhibitors, pyrazole 3 (Pyr3) is most selective and potent. However, Pyr3 only provides limited benefits in pilocarpine-treated mice, likely due to its low metabolic stability and potential toxicity. We recently reported a modified pyrazole compound 20 (or JW-65) that has improved stability and safety. The objective of this study was to explore the effects of TRPC3 inhibition by our current lead compound JW-65 on seizure susceptibility.
Methods: We first examined the pharmacokinetic properties including plasma half-life and brain to plasma ratio of JW-65 after systemic administration in mice. We then investigated the effects of TRPC3 inhibition by JW-65 on behavioral and electrographic seizures in mice treated with pilocarpine. To ensure our findings are not model specific, we assessed the susceptibility of JW-65-treated mice to pentylenetetrazole (PTZ)-induced seizures with phenytoin as a comparator.
Results: JW-65 showed adequate half-life and brain penetration in mice, justifying its use for central nervous system conditions. Systemic treatment with JW-65 before pilocarpine injection in mice markedly impaired the initiation of behavioral seizures. This antiseizure action was recapitulated when JW-65 was administered after pilocarpine-induced behavioral seizures were well established and was confirmed by time-locked electroencephalographic monitoring and synchronized video. Moreover, JW-65-treated mice showed substantially decreased susceptibility to PTZ-induced seizures in a dose-dependent manner.
Significance: These results suggest that pharmacological inhibition of the TRPC3 channels by our novel compound JW-65 might represent a new antiseizure strategy engaging a previously undrugged mechanism of action. Hence, this proof-of-concept study establishes TRPC3 as a novel feasible therapeutic target for the treatment of some forms of epilepsy.
Keywords: calcium channels; pentylenetetrazole; pilocarpine; seizure; status epilepticus; transient receptor potential channels.
© 2022 International League Against Epilepsy.
Conflict of interest statement
Conflict of Interest
None of the authors have any conflict of interest to disclose. The authors confirm that they have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Figures



Similar articles
-
TRPC3 channels play a critical role in the theta component of pilocarpine-induced status epilepticus in mice.Epilepsia. 2017 Feb;58(2):247-254. doi: 10.1111/epi.13648. Epub 2016 Dec 24. Epilepsia. 2017. PMID: 28012173 Free PMC article.
-
Inhibition of TRPC3 channels suppresses seizure susceptibility in the genetically-epilepsy prone rats.Eur J Pharmacol. 2024 Aug 15;977:176722. doi: 10.1016/j.ejphar.2024.176722. Epub 2024 Jun 6. Eur J Pharmacol. 2024. PMID: 38851562
-
Effects of acute administration of 4-allyl-2,6-dimethoxyphenol in mouse models of seizures.Epilepsy Res. 2024 Sep;205:107421. doi: 10.1016/j.eplepsyres.2024.107421. Epub 2024 Jul 26. Epilepsy Res. 2024. PMID: 39068729
-
Review: cholinergic mechanisms and epileptogenesis. The seizures induced by pilocarpine: a novel experimental model of intractable epilepsy.Synapse. 1989;3(2):154-71. doi: 10.1002/syn.890030207. Synapse. 1989. PMID: 2648633 Review.
-
[Pharmacological properties of novel TRPC channel inhibitors].Yakugaku Zasshi. 2010 Mar;130(3):303-11. doi: 10.1248/yakushi.130.303. Yakugaku Zasshi. 2010. PMID: 20190514 Review. Japanese.
Cited by
-
Transient inhibition of microsomal prostaglandin E synthase-1 after status epilepticus blunts brain inflammation and is neuroprotective.Mol Brain. 2023 Jan 25;16(1):14. doi: 10.1186/s13041-023-01008-y. Mol Brain. 2023. PMID: 36694204 Free PMC article.
-
Pharmacologically targeting transient receptor potential channels for seizures and epilepsy: Emerging preclinical evidence of druggability.Pharmacol Ther. 2023 Apr;244:108384. doi: 10.1016/j.pharmthera.2023.108384. Epub 2023 Mar 16. Pharmacol Ther. 2023. PMID: 36933703 Free PMC article. Review.
-
Transient Receptor Potential (TRP) Channels in Pain, Neuropsychiatric Disorders, and Epilepsy.Int J Mol Sci. 2023 Mar 1;24(5):4714. doi: 10.3390/ijms24054714. Int J Mol Sci. 2023. PMID: 36902145 Free PMC article. Review.
-
TRPC channels as emerging targets for seizure disorders.Trends Pharmacol Sci. 2022 Sep;43(9):787-798. doi: 10.1016/j.tips.2022.06.007. Epub 2022 Jul 12. Trends Pharmacol Sci. 2022. PMID: 35840362 Free PMC article. Review.
-
TRP (transient receptor potential) ion channel family: structures, biological functions and therapeutic interventions for diseases.Signal Transduct Target Ther. 2023 Jul 5;8(1):261. doi: 10.1038/s41392-023-01464-x. Signal Transduct Target Ther. 2023. PMID: 37402746 Free PMC article. Review.
References
-
- Devinsky O, Vezzani A, O'Brien TJ, Jette N, Scheffer IE, de Curtis M, et al. Epilepsy. Nat Rev Dis Primers. 2018;4:18024. - PubMed
-
- Carvill GL, Dulla CG, Lowenstein DH, Brooks-Kayal AR. The path from scientific discovery to cures for epilepsy. Neuropharmacology. 2020;167:107702. - PubMed
-
- Sills GJ, Rogawski MA. Mechanisms of action of currently used antiseizure drugs. Neuropharmacology. 2020;168:107966. - PubMed
-
- Löscher W, Klein P. The feast and famine: Epilepsy treatment and treatment gaps in early 21st century. Neuropharmacology. 2020;170:108055. - PubMed
-
- Janmohamed M, Brodie MJ, Kwan P. Pharmacoresistance - Epidemiology, mechanisms, and impact on epilepsy treatment. Neuropharmacology. 2020;168:107790. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

