Meta-Analysis of APP Expression Modulated by SARS-CoV-2 Infection via the ACE2 Receptor
- PMID: 35163117
- PMCID: PMC8835589
- DOI: 10.3390/ijms23031182
Meta-Analysis of APP Expression Modulated by SARS-CoV-2 Infection via the ACE2 Receptor
Abstract
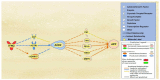
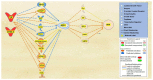
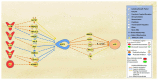
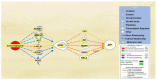
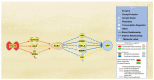
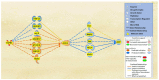
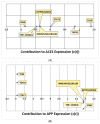
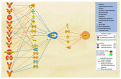
Alzheimer's disease (AD) is characterized by the deposition of amyloid-beta (Aβ) plaques from improper amyloid-beta precursor protein (APP) cleavage. Following studies of inflammation caused by coronavirus-2019 (COVID-19) infection, this study investigated the impact of COVID-19 on APP expression. A meta-analysis was conducted utilizing QIAGEN Ingenuity Pathway Analysis (IPA) to examine the link between severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) and the modulation of APP expression upon virus binding the Angiotensin-converting enzyme-2 (ACE2) receptor. A Core Analysis was run on the infection by severe acute respiratory syndrome (SARS) coronavirus node, which included molecules affected by SARS-CoV-2, revealing its upstream regulators. Intermediary molecules were found between the upstream regulators and ACE2 and between ACE2 and APP. Activation of the upstream regulators downregulated the expression of ACE2 with a Z-score of -1.719 (p-value = 0.086) and upregulated APP with a Z-score of 1.898 (p-value = 0.058), showing a less than 10% chance of the results occurring by chance and pointing to an inverse relationship between ACE2 and APP expression. The neuroinflammation signaling pathway was the fifth top canonical pathway involved in APP upregulation. The study results suggest that ACE2 could be downregulated by SARS-CoV-2, resulting in APP upregulation, and potentially exacerbating the onset and progression of AD.
Keywords: Alzheimer’s disease; amyloid-beta precursor protein; angiotensin-converting enzyme-2; coronavirus-2019; meta-analysis; severe acute respiratory syndrome-coronavirus-2.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Network Meta-analysis on the Changes of Amyloid Precursor Protein Expression Following SARS-CoV-2 Infection.J Neuroimmune Pharmacol. 2021 Dec;16(4):756-769. doi: 10.1007/s11481-021-10012-9. Epub 2021 Nov 10. J Neuroimmune Pharmacol. 2021. PMID: 34757528 Free PMC article.
-
Meta-Analysis of the Mechanisms Underlying COVID-19 Modulation of Parkinson's Disease.Int J Mol Sci. 2023 Aug 31;24(17):13554. doi: 10.3390/ijms241713554. Int J Mol Sci. 2023. PMID: 37686360 Free PMC article.
-
Protein Expression of Angiotensin-Converting Enzyme 2 (ACE2) is Upregulated in Brains with Alzheimer's Disease.Int J Mol Sci. 2021 Feb 8;22(4):1687. doi: 10.3390/ijms22041687. Int J Mol Sci. 2021. PMID: 33567524 Free PMC article.
-
Angiotensin-Converting Enzyme 2 (ACE2) in the Pathogenesis of ARDS in COVID-19.Front Immunol. 2021 Dec 22;12:732690. doi: 10.3389/fimmu.2021.732690. eCollection 2021. Front Immunol. 2021. PMID: 35003058 Free PMC article. Review.
-
SARS-CoV-2, ACE2 expression, and systemic organ invasion.Physiol Genomics. 2021 Feb 1;53(2):51-60. doi: 10.1152/physiolgenomics.00087.2020. Epub 2020 Dec 4. Physiol Genomics. 2021. PMID: 33275540 Free PMC article. Review.
Cited by
-
Amyloidogenic proteins in the SARS-CoV and SARS-CoV-2 proteomes.Nat Commun. 2023 Feb 20;14(1):945. doi: 10.1038/s41467-023-36234-4. Nat Commun. 2023. PMID: 36806058 Free PMC article.
-
Amyloid precursor protein facilitates SARS-CoV-2 virus entry into cells and enhances amyloid-β-associated pathology in APP/PS1 mouse model of Alzheimer's disease.Transl Psychiatry. 2023 Dec 16;13(1):396. doi: 10.1038/s41398-023-02692-z. Transl Psychiatry. 2023. PMID: 38104129 Free PMC article.
-
Deciphering infected cell types, hub gene networks and cell-cell communication in infectious bronchitis virus via single-cell RNA sequencing.PLoS Pathog. 2024 May 14;20(5):e1012232. doi: 10.1371/journal.ppat.1012232. eCollection 2024 May. PLoS Pathog. 2024. PMID: 38743760 Free PMC article.
-
The viral hypothesis in Alzheimer's disease: SARS-CoV-2 on the cusp.Front Aging Neurosci. 2023 Mar 15;15:1129640. doi: 10.3389/fnagi.2023.1129640. eCollection 2023. Front Aging Neurosci. 2023. PMID: 37009449 Free PMC article. Review.
-
Neurological, psychological, psychosocial complications of long-COVID and their management.Neurol Sci. 2025 Jan;46(1):1-23. doi: 10.1007/s10072-024-07854-5. Epub 2024 Nov 9. Neurol Sci. 2025. PMID: 39516425 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

