Neutralization against Omicron SARS-CoV-2 from previous non-Omicron infection
- PMID: 35140233
- PMCID: PMC8828871
- DOI: 10.1038/s41467-022-28544-w
Neutralization against Omicron SARS-CoV-2 from previous non-Omicron infection
Abstract
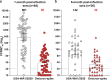
The spread of the Omicron SARS-CoV-2 variant underscores the importance of analyzing the cross-protection from previous non-Omicron infection. We have developed a high-throughput neutralization assay for Omicron SARS-CoV-2 by engineering the Omicron spike gene into an mNeonGreen USA-WA1/2020 SARS-CoV-2 (isolated in January 2020). Using this assay, we determine the neutralization titers (defined as the maximal serum dilution that inhibited 50% of infectious virus) of patient sera collected at 1- or 6-months after infection with non-Omicron SARS-CoV-2. From 1- to 6-month post-infection, the neutralization titers against USA-WA1/2020 decrease from 601 to 142 (a 4.2-fold reduction), while the neutralization titers against Omicron-spike SARS-CoV-2 remain low at 38 and 32, respectively. Thus, at 1- and 6-months after non-Omicron SARS-CoV-2 infection, the neutralization titers against Omicron are 15.8- and 4.4-fold lower than those against USA-WA1/2020, respectively. The low cross-neutralization against Omicron from previous non-Omicron infection supports vaccination of formerly infected individuals to mitigate the health impact of the ongoing Omicron surge.
© 2022. The Author(s).
Conflict of interest statement
The authors declare competing interests. X.X. and P.-Y.S. have filed a patent on the reverse genetic system. J.Z., H.X., X.X., and P.-Y.S. received compensation from Pfizer for COVID-19 vaccine development. Other authors declare no competing interests.
Figures
Similar articles
-
Cross-neutralization of Omicron BA.1 against BA.2 and BA.3 SARS-CoV-2.Nat Commun. 2022 May 26;13(1):2956. doi: 10.1038/s41467-022-30580-5. Nat Commun. 2022. PMID: 35618703 Free PMC article.
-
Considerable escape of SARS-CoV-2 Omicron to antibody neutralization.Nature. 2022 Feb;602(7898):671-675. doi: 10.1038/s41586-021-04389-z. Epub 2021 Dec 23. Nature. 2022. PMID: 35016199
-
Wild-type SARS-CoV-2 neutralizing immunity decreases across variants and over time but correlates well with diagnostic testing.Front Immunol. 2023 Feb 8;14:1055429. doi: 10.3389/fimmu.2023.1055429. eCollection 2023. Front Immunol. 2023. PMID: 36845123 Free PMC article.
-
Long-term, infection-acquired immunity against the SARS-CoV-2 Delta variant in a hamster model.Cell Rep. 2022 Feb 15;38(7):110394. doi: 10.1016/j.celrep.2022.110394. Epub 2022 Jan 31. Cell Rep. 2022. PMID: 35139368 Free PMC article.
-
mRNA-1273 and BNT162b2 mRNA vaccines have reduced neutralizing activity against the SARS-CoV-2 omicron variant.Cell Rep Med. 2022 Jan 24;3(2):100529. doi: 10.1016/j.xcrm.2022.100529. eCollection 2022 Feb 15. Cell Rep Med. 2022. PMID: 35233550 Free PMC article.
Cited by
-
Development and evaluation of vaccination strategies for addressing the continuous evolution SARS-CoV-2 based on recombinant trimeric protein technology: Potential for cross-neutralizing activity and broad coronavirus response.Heliyon. 2024 Jul 17;10(14):e34492. doi: 10.1016/j.heliyon.2024.e34492. eCollection 2024 Jul 30. Heliyon. 2024. PMID: 39148990 Free PMC article.
-
SARS-CoV-2 Omicron: Light at the End of the Long Pandemic Tunnel or Another False Dawn for Immunodeficient Patients?J Allergy Clin Immunol Pract. 2022 Sep;10(9):2267-2273. doi: 10.1016/j.jaip.2022.06.011. Epub 2022 Jun 22. J Allergy Clin Immunol Pract. 2022. PMID: 35752434 Free PMC article.
-
Antibody Levels Poorly Reflect on the Frequency of Memory B Cells Generated following SARS-CoV-2, Seasonal Influenza, or EBV Infection.Cells. 2022 Nov 18;11(22):3662. doi: 10.3390/cells11223662. Cells. 2022. PMID: 36429090 Free PMC article.
-
Diphyllin elicits a doubled-pronged attack on the entry of SARS-CoV-2 by inhibiting cathepsin L and furin.Virus Res. 2024 Dec;350:199485. doi: 10.1016/j.virusres.2024.199485. Epub 2024 Oct 19. Virus Res. 2024. PMID: 39424146 Free PMC article.
-
The arrival of SARS-CoV-2-neutralizing antibodies in a currently available commercial immunoglobulin.J Allergy Clin Immunol. 2022 Jun;149(6):1958-1959. doi: 10.1016/j.jaci.2022.03.026. Epub 2022 Apr 22. J Allergy Clin Immunol. 2022. PMID: 35465974 Free PMC article. No abstract available.
References
-
- WHO. Classification of Omicron (B.1.1.529): SARS-CoV-2 Variant of Concern. https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1.... (2021).
-
- UK Health Security Agency. SARS-CoV-2 variants of concern and variants under investigation in England. Technical brief 31 (2021).
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
- R01 AI134907/AI/NIAID NIH HHS/United States
- HHSN272201600013C/AI/NIAID NIH HHS/United States
- R24 AI120942/AI/NIAID NIH HHS/United States
- R43 AI145617/AI/NIAID NIH HHS/United States
- HHSN272201600013C, AI134907, AI145617, UL1TR001439/U.S. Department of Health & Human Services | NIH | Center for Information Technology (Center for Information Technology, National Institutes of Health)
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

