Transient receptor potential V1 modulates neuroinflammation in Parkinson's disease dementia: Molecular implications for electroacupuncture and rivastigmine
- PMID: 35096291
- PMCID: PMC8769514
- DOI: 10.22038/IJBMS.2021.56156.12531
Transient receptor potential V1 modulates neuroinflammation in Parkinson's disease dementia: Molecular implications for electroacupuncture and rivastigmine
Abstract
Objectives: Parkinson's disease (PD) is a common progressive neurodegeneration disease. Its incidence increases with age and affects about 1% of people over 60. Incidentally, transient receptor potential V1 (TRPV1) and its relation with neuroinflammation in mouse brain has been widely reported.
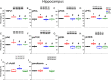
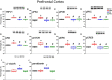
Materials and methods: We used 6-hydroxydopamine (6-OHDA) to induce PDD in mice. We then used the Morris water maze and Bio-Plex to test learning and inflammatory mediators in mouse plasma. Western blotting and immunostaining were used to examine TRPV1 pathway in the hippocampus and medial prefrontal cortex (mPFC).
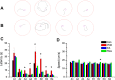
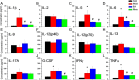
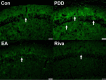
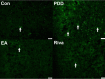
Results: On acquisition days 3 (Control = 4.40 ± 0.8 sec, PDD = 9.82 ± 1.52 sec, EA = 5.04 ± 0.58 sec, Riva = 4.75 ± 0.87 sec; P=0.001) and 4, reversal learning days 1, 2, 3 (Control = 2.86 ± 0.46 sec, PDD = 9.80 ± 1.83 sec, EA = 4.6 ± 0.82 sec, Riva = 4.6 ± 1.03 sec; P=0.001) and 4, PDD mice showed significantly longer escape latency than the other three groups. Results showed that several cytokines were up-regulated in PDD mice and reversed by EA and rivastigmine. TRPV1 and downstream molecules were up-regulated in PDD mice and further reversed by EA and rivastigmine. Interestingly, α7 nicotinic receptors and parvalbumin levels in both the hippocampus and prefrontal cortex increased in EA-treated mice, but not in rivastigmine-treated mice.
Conclusion: Our results showed that TRPV1 played a role in the modulation of neuroinflammation of PDD, and could potentially be a new target for treatment.
Keywords: Electroacupuncture; Hippocampus; Neuroinflammation; Parkinson’s disease – dementia; Rivastigmine; Transient receptor potential- V1.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Transient receptor potential V1 (TRPV1) modulates the therapeutic effects for comorbidity of pain and depression: The common molecular implication for electroacupuncture and omega-3 polyunsaturated fatty acids.Brain Behav Immun. 2020 Oct;89:604-614. doi: 10.1016/j.bbi.2020.06.033. Epub 2020 Jul 5. Brain Behav Immun. 2020. PMID: 32640285
-
Electroacupuncture Attenuates Chronic Inflammatory Pain and Depression Comorbidity through Transient Receptor Potential V1 in the Brain.Am J Chin Med. 2021;49(6):1417-1435. doi: 10.1142/S0192415X2150066X. Epub 2021 Jul 3. Am J Chin Med. 2021. PMID: 34224338
-
Rivastigmine for the treatment of dementia associated with Parkinson's disease.Neuropsychiatr Dis Treat. 2007 Dec;3(6):775-83. doi: 10.2147/ndt.s1134. Neuropsychiatr Dis Treat. 2007. PMID: 19300613 Free PMC article.
-
Cholinesterase inhibitors for dementia with Lewy bodies, Parkinson's disease dementia and cognitive impairment in Parkinson's disease.Cochrane Database Syst Rev. 2012 Mar 14;2012(3):CD006504. doi: 10.1002/14651858.CD006504.pub2. Cochrane Database Syst Rev. 2012. PMID: 22419314 Free PMC article. Review.
-
Rivastigmine in dementia associated with Parkinson's disease and Alzheimer's disease: similarities and differences.J Alzheimers Dis. 2007 Jul;11(4):509-19. doi: 10.3233/jad-2007-11412. J Alzheimers Dis. 2007. PMID: 17656830 Review.
Cited by
-
Electroacupuncture Reduces Fibromyalgia Pain via Neuronal/Microglial Inactivation and Toll-like Receptor 4 in the Mouse Brain: Precise Interpretation of Chemogenetics.Biomedicines. 2024 Feb 7;12(2):387. doi: 10.3390/biomedicines12020387. Biomedicines. 2024. PMID: 38397989 Free PMC article.
-
Anti-Inflammatory Effect of Traditional Chinese Medicine on the Concept of Mind-Body Interface.Adv Exp Med Biol. 2023;1411:435-458. doi: 10.1007/978-981-19-7376-5_19. Adv Exp Med Biol. 2023. PMID: 36949321 Review.
-
TRP (transient receptor potential) ion channel family: structures, biological functions and therapeutic interventions for diseases.Signal Transduct Target Ther. 2023 Jul 5;8(1):261. doi: 10.1038/s41392-023-01464-x. Signal Transduct Target Ther. 2023. PMID: 37402746 Free PMC article. Review.
-
A Systematic Review of the Effects of Capsaicin on Alzheimer's Disease.Int J Mol Sci. 2023 Jun 15;24(12):10176. doi: 10.3390/ijms241210176. Int J Mol Sci. 2023. PMID: 37373321 Free PMC article. Review.
References
-
- Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG. The Sydney multicenter study of Parkinson’s disease: the inevitability of dementia at 20 years. Mov Disord. 2008;23:837–844. - PubMed
-
- Kehagia AA, Barker RA, Robbins TW. Neuropsychological and clinical heterogeneity of cognitive impairment and dementia in patients with Parkinson’s disease. Lancet Neurol. 2010;9:1200–1213. - PubMed
-
- Diaz NL, Waters CH. Current strategies in the treatment of Parkinson’s disease and a personalized approach to management. Expert Rev Neurother. 2009;9:1781–1789. - PubMed
LinkOut - more resources
Full Text Sources
