Interleukin-1 blocking agents for treating COVID-19
- PMID: 35080773
- PMCID: PMC8791232
- DOI: 10.1002/14651858.CD015308
Interleukin-1 blocking agents for treating COVID-19
Abstract
Background: Interleukin-1 (IL-1) blocking agents have been used for treating severe coronavirus disease 2019 (COVID-19), on the premise that their immunomodulatory effect might be beneficial in people with COVID-19.
Objectives: To assess the effects of IL-1 blocking agents compared with standard care alone or with placebo on effectiveness and safety outcomes in people with COVID-19. We will update this assessment regularly.
Search methods: We searched the Cochrane COVID-19 Study Register and the COVID-19 L-OVE Platform (search date 5 November 2021). These sources are maintained through regular searches of MEDLINE, Embase, CENTRAL, trial registers and other sources. We also checked the World Health Organization International Clinical Trials Registry Platform, regulatory agency websites, Retraction Watch (search date 3 November 2021).
Selection criteria: We included randomised controlled trials (RCTs) evaluating IL-1 blocking agents compared with standard care alone or with placebo for people with COVID-19, regardless of disease severity.
Data collection and analysis: We followed Cochrane methodology. The protocol was amended to reduce the number of outcomes considered. Two researchers independently screened and extracted data and assessed the risk of bias with the Cochrane Risk of Bias 2 tool. We rated the certainty of evidence using the GRADE approach for the critical outcomes of clinical improvement (Day 28; ≥ D60); WHO Clinical Progression Score of level 7 or above (i.e. the proportion of participants with mechanical ventilation +/- additional organ support OR death) (D28; ≥ D60); all-cause mortality (D28; ≥ D60); incidence of any adverse events; and incidence of serious adverse events.
Main results: We identified four RCTs of anakinra (three published in peer-reviewed journals, one reported as a preprint) and two RCTs of canakinumab (published in peer-reviewed journals). All trials were multicentre (2 to 133 centres). Two trials stopped early (one due to futility and one as the trigger for inferiority was met). The median/mean age range varied from 58 to 68 years; the proportion of men varied from 58% to 77%. All participants were hospitalised; 67% to 100% were on oxygen at baseline but not intubated; between 0% and 33% were intubated at baseline. We identified a further 16 registered trials with no results available, of which 15 assessed anakinra (four completed, four terminated, five ongoing, three not recruiting) and one (completed) trial assessed canakinumab. Effectiveness of anakinra for people with COVID-19 Anakinra probably results in little or no increase in clinical improvement at D28 (risk ratio (RR) 1.08, 95% confidence interval (CI) 0.97 to 1.20; 3 RCTs, 837 participants; absolute effect: 59 more per 1000 (from 22 fewer to 147 more); moderate-certainty evidence. The evidence is uncertain about an effect of anakinra on 1) the proportion of participants with a WHO Clinical Progression Score of level 7 or above at D28 (RR 0.67, 95% CI 0.36 to 1.22; 2 RCTs, 722 participants; absolute effect: 55 fewer per 1000 (from 107 fewer to 37 more); low-certainty evidence) and ≥ D60 (RR 0.54, 95% CI 0.30 to 0.96; 1 RCT, 606 participants; absolute effect: 47 fewer per 1000 (from 72 fewer to 4 fewer) low-certainty evidence); and 2) all-cause mortality at D28 (RR 0.69, 95% CI 0.34 to 1.39; 2 RCTs, 722 participants; absolute effect: 32 fewer per 1000 (from 68 fewer to 40 more); low-certainty evidence). The evidence is very uncertain about an effect of anakinra on 1) the proportion of participants with clinical improvement at ≥ D60 (RR 0.93, 95% CI 0.78 to 1.12; 1 RCT, 115 participants; absolute effect: 59 fewer per 1000 (from 186 fewer to 102 more); very low-certainty evidence); and 2) all-cause mortality at ≥ D60 (RR 1.03, 95% CI 0.68 to 1.56; 4 RCTs, 1633 participants; absolute effect: 8 more per 1000 (from 84 fewer to 147 more); very low-certainty evidence). Safety of anakinra for people with COVID-19 Anakinra probably results in little or no increase in adverse events (RR 1.02, 95% CI 0.94 to 1.11; 2 RCTs, 722 participants; absolute effect: 14 more per 1000 (from 43 fewer to 78 more); moderate-certainty evidence). The evidence is uncertain regarding an effect of anakinra on serious adverse events (RR 0.95, 95% CI 0.58 to 1.56; 2 RCTs, 722 participants; absolute effect: 12 fewer per 1000 (from 104 fewer to 138 more); low-certainty evidence). Effectiveness of canakinumab for people with COVID-19 Canakinumab probably results in little or no increase in clinical improvement at D28 (RR 1.05, 95% CI 0.96 to 1.14; 2 RCTs, 499 participants; absolute effect: 42 more per 1000 (from 33 fewer to 116 more); moderate-certainty evidence). The evidence of an effect of canakinumab is uncertain on 1) the proportion of participants with a WHO Clinical Progression Score of level 7 or above at D28 (RR 0.72, 95% CI 0.44 to 1.20; 2 RCTs, 499 participants; absolute effect: 35 fewer per 1000 (from 69 fewer to 25 more); low-certainty evidence); and 2) all-cause mortality at D28 (RR:0.75; 95% CI 0.39 to 1.42); 2 RCTs, 499 participants; absolute effect: 20 fewer per 1000 (from 48 fewer to 33 more); low-certainty evidence). The evidence is very uncertain about an effect of canakinumab on all-cause mortality at ≥ D60 (RR 0.55, 95% CI 0.16 to 1.91; 1 RCT, 45 participants; absolute effect: 112 fewer per 1000 (from 210 fewer to 227 more); very low-certainty evidence). Safety of canakinumab for people with COVID-19 Canakinumab probably results in little or no increase in adverse events (RR 1.02; 95% CI 0.86 to 1.21; 1 RCT, 454 participants; absolute effect: 11 more per 1000 (from 74 fewer to 111 more); moderate-certainty evidence). The evidence of an effect of canakinumab on serious adverse events is uncertain (RR 0.80, 95% CI 0.57 to 1.13; 2 RCTs, 499 participants; absolute effect: 44 fewer per 1000 (from 94 fewer to 28 more); low-certainty evidence).
Authors' conclusions: Overall, we did not find evidence for an important beneficial effect of IL-1 blocking agents. The evidence is uncertain or very uncertain for several outcomes. Sixteen trials of anakinra and canakinumab with no results are currently registered, of which four are completed, and four terminated. The findings of this review are updated on the COVID-NMA platform (covid-nma.com).
Trial registration: ClinicalTrials.gov NCT04330638.
Copyright © 2022 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
Mauricia Davidson: none known.
Sonia Menon: works as systematic reviewer for p95 consultancy company.
Anna Chaimani: none known.
Theodoros Evrenoglou: none known.
Lina Ghosn: none known.
Carolina Graña: none known.
Nicholas Henschke: is employed by Cochrane Response, an evidence consultancy initiative from Cochrane. Cochrane Response was commissioned by the WHO to perform work on the living systematic review and living network meta‐analysis for COVID‐19 studies.
Elise Cogo: is employed by Cochrane Response, an evidence consultancy initiative from Cochrane. Cochrane Response was commissioned by the WHO to perform work on the living systematic review and living network meta‐analysis for COVID‐19 studies.
Gemma Villanueva: is employed by Cochrane Response, an evidence consultancy initiative from Cochrane. Cochrane Response was commissioned by the WHO to perform work on the living systematic review and living network meta‐analysis for COVID‐19 studies.
Gabriel Ferrand: none known.
Carolina Riveros: none known.
Philipp Kapp: none known.
Hillary Bonnet: none known.
Conor Moran: none known.
Declan Devane: works for Cochrane Ireland and Evidence Synthesis Ireland which are funded within the National University of Ireland Galway (Ireland) by the Health Research Board (HRB) and the Health and Social Care, Research and Development (HSC R&D) Division of the Public Health Agency in Northern Ireland.
Joerg J Meerpohl: reports funding from the Federal Ministry of Health and the Federal Ministry of Education and Research.
Gabriel Rada: none known.
Asbjørn Hróbjartsson: none known.
Giacomo Grasselli: receives personal fees for lectures from Getinge, Fisher&Paykel, Draeger Medical, Biotest, Thermofisher and MSD; support for travel‐meeting expenses from Biotest and Getinge (all outside the present work). GG also received an unrestricted research grant from Fisher&Paykel (unrelated to the present work).
David Tovey: has a part‐time paid consultancy with the Université de Paris.
Philippe Ravaud: is a minority shareholder of INATO. PR was the methodologist of the CORIMUNO‐19 platform which generated the Mariette CORIMUNO‐19 Collaborative 2021 trial. PR did not undertake any inclusion decisions/data extraction or risk of bias assessments for the Mariette CORIMUNO‐19 Collaborative 2021 trial.
Isabelle Boutron: is director of Cochrane France and co‐convenor of the Cochrane Bias methods group.
Figures
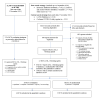
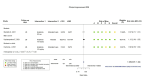
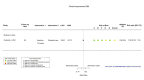

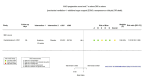
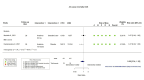

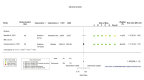
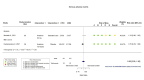


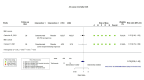
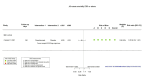
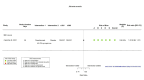
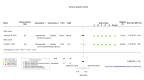


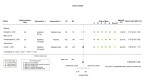



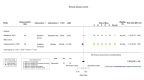


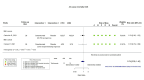
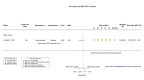
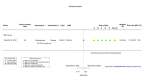
Similar articles
-
Interleukin-6 blocking agents for treating COVID-19: a living systematic review.Cochrane Database Syst Rev. 2021 Mar 18;3(3):CD013881. doi: 10.1002/14651858.CD013881. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2023 Jun 1;6:CD013881. doi: 10.1002/14651858.CD013881.pub2. PMID: 33734435 Free PMC article. Updated.
-
Interleukin-6 blocking agents for treating COVID-19: a living systematic review.Cochrane Database Syst Rev. 2023 Jun 1;6(6):CD013881. doi: 10.1002/14651858.CD013881.pub2. Cochrane Database Syst Rev. 2023. PMID: 37260086 Free PMC article. Review.
-
Remdesivir for the treatment of COVID-19.Cochrane Database Syst Rev. 2023 Jan 25;1(1):CD014962. doi: 10.1002/14651858.CD014962.pub2. Cochrane Database Syst Rev. 2023. PMID: 36695483 Free PMC article. Review.
-
Remdesivir for the treatment of COVID-19.Cochrane Database Syst Rev. 2021 Aug 5;8(8):CD014962. doi: 10.1002/14651858.CD014962. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2023 Jan 25;1:CD014962. doi: 10.1002/14651858.CD014962.pub2. PMID: 34350582 Free PMC article. Updated.
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
Cited by
-
COVID-19: An Update on Epidemiology, Prevention and Treatment, September-2023.Infect Dis Clin Microbiol. 2023 Sep 30;5(3):165-187. doi: 10.36519/idcm.2023.251. eCollection 2023 Sep. Infect Dis Clin Microbiol. 2023. PMID: 38633552 Free PMC article. Review.
-
Interleukin-1 Blockers: A Paradigm Shift in the Treatment of Recurrent Pericarditis.Life (Basel). 2024 Feb 26;14(3):305. doi: 10.3390/life14030305. Life (Basel). 2024. PMID: 38541631 Free PMC article. Review.
-
Immunomodulators for immunocompromised patients hospitalized for COVID-19: a meta-analysis of randomized controlled trials.EClinicalMedicine. 2024 Feb 9;69:102472. doi: 10.1016/j.eclinm.2024.102472. eCollection 2024 Mar. EClinicalMedicine. 2024. PMID: 38361992 Free PMC article.
-
Rethinking IL-1 Antagonism in Respiratory Viral Infections: A Role for IL-1 Signaling in the Development of Antiviral T Cell Immunity.Int J Mol Sci. 2023 Oct 30;24(21):15770. doi: 10.3390/ijms242115770. Int J Mol Sci. 2023. PMID: 37958758 Free PMC article.
-
Anakinra authorized to treat severe coronavirus disease 2019; Sepsis breakthrough or time to reflect?Front Microbiol. 2023 Oct 19;14:1250483. doi: 10.3389/fmicb.2023.1250483. eCollection 2023. Front Microbiol. 2023. PMID: 37928695 Free PMC article.
References
References to studies included in this review
Caricchio CAN‐COVID 2021 {published and unpublished data}
-
- Caricchio R, Abbate A, Gordeev I, Meng J, Hsue PY, Neogi T, et al, CAN-COVID Investigators.Effect of canakinumab vs placebo on survival without invasive mechanical ventilation in patients hospitalized with severe COVID-19: a randomized clinical trial. JAMA 2021;326(3):230-9. [DOI: 10.1001/jama.2021.9508] - DOI - PMC - PubMed
Cremer Three C Study 2021 {published data only}
-
- Cremer PC, Sheng CC, Sahoo D, Dugar S, Prada RA, Wang KM et al, the Three C study group.Double-blind randomized proof-of-concept trial of canakinumab in patients with COVID-19 associated cardiac injury and heightened inflammation. European Heart Journal Open 2021;43(10):1055-63. [DOI: 10.1002/clc.23451] - DOI - PMC - PubMed
Declercq COV‐AID 2021 {published data only (unpublished sought but not used)}
-
- Declercq J, Van Damme KF, Leeuw ED, Maes B, Bosteels C, Tavernier SJ, et al.Effect of anti-interleukin drugs in patients with COVID-19 and signs of cytokine release syndrome (COV-AID): a factorial, randomised, controlled trial. Lancet. Respiratory Medicine 2021;9(12):1427-38. [DOI: 10.1016/S2213-2600(21)00377-5] - DOI - PMC - PubMed
Derde REMAP‐CAP 2021 {published data only (unpublished sought but not used)}
-
- Derde LP, The REMAP-CAP Investigators.Effectiveness of tocilizumab, sarilumab, and anakinra for critically ill patients with COVID-19. The REMAP-CAP COVID-19 immune modulation therapy domain randomized clinical trial. medRxiv 2021. [DOI: 10.1101/2021.06.18.21259133] - DOI
Kyriazopoulou SAVE‐MORE 2021 {published and unpublished data}
-
- Kyriazopoulou E, Poulakou G, Milionis H, Metallidis S, Adamis G, Tsiakos K, et al.Early anakinra treatment for COVID-19 guided by urokinase plasminogen receptor. medRxiv [Preprint] 2021. [DOI: 10.1101/2021.05.16.21257283] - DOI
-
- Kyriazopoulou E, Poulakou G, Milionis H, Metallidis S, Adamis G, Tsiakos K, et al.Early treatment of COVID-19 with anakinra guided by soluble urokinase plasminogen receptor plasma levels: a double-blind, randomized controlled phase 3 trial. Nature Medicine 2021;27(10):1752-60. [DOI: 10.1038/s41591-021-01499-z] - DOI - PMC - PubMed
Mariette CORIMUNO‐19 Collaborative 2021 {published and unpublished data}
-
- Mariette X, the CORIMUNO-19 Collaborative group.Effect of anakinra versus usual care in adults in hospital with COVID-19 and mild-to-moderate pneumonia (CORIMUNO-ANA-1): a randomised controlled trial. Lancet. Respiratory Medicine 2021;9(3):295-304. [DOI: 10.1016/S2213-2600(20)30556-7] - DOI - PMC - PubMed
Additional references
Attaway 2021
-
- Attaway AH, Scheraga RG, Bhimraj A, Biehl M, Hatipoğlu U.Severe covid-19 pneumonia: pathogenesis and clinical management. BMJ 2021;372:n436. - PubMed
Barkas 2021
Boutron 2020a
Boutron 2020b
-
- Boutron I, Chaimani A, Devane D, Meerpohl JJ, Rada G, Hróbjartsson A, et al.Interventions for the prevention and treatment of COVID-19: a living mapping of research and living network meta-analysis. Cochrane Database of Systematic Reviews 2020, Issue 11. Art. No: CD013769. [DOI: 10.1002/14651858.CD013769] - DOI
Boutron 2020c
-
- Boutron I, Chaimani A, Ghosn L.Interleukin (IL)-1 blocking agents for the treatment of COVID-19 A living systematic review. Zenodo 23 October 2020. [DOI: 10.5281/zenodo.5698219] - DOI
Boutron 2021
-
- Boutron I, Chaimani A, Ghosn L.Interleukin (IL)-1 blocking agents for the treatment of COVID-19 A living systematic review. Zenodo 8 November 2021. [DOI: 10.5281/zenodo.5698123] - DOI
Cabanac 2021
Cantini 2020
Cavalli 2021
Chaimani 2018
Davidson 2021
-
- Davidson M, Menon S, Chaimani A, Evrenoglou T, Ghosn L, Graña C, et al.Dataset: Interleukin (IL)-1 blocking agents for the treatment of COVID-19 A living systematic review. Zenodo 15 January 2022. [DOI: 10.5281/zenodo.5853927] - DOI
Dinarello 2012
GRADEpro GDT [Computer program]
-
- McMaster University (developed by Evidence Prime) GRADEpro GDT.Hamilton (ON): McMaster University (developed by Evidence Prime), accessed 21 February 2021. Available at gradepro.org.
Higgins 2021
-
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s).Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Horby 2021
-
- Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L et al.Dexamethasone in Hospitalized Patients with Covid-19. New England Journal of Medicine 25 February 2021;384(8):693-704.
Juul 2020a
-
- Juul S, Nielsen EE, Feinberg J, Siddiqui F, Jørgensen CK, Barot E, et al.Interventions for treatment of COVID-19: a living systematic review with meta-analyses and trial sequential analyses (The LIVING Project). PLOS Medicine 2020;17(9):e1003293. Erratum in: PLoS Med. 2020 Dec 29;17(12):e1003517. - PMC - PubMed
Juul 2020b
-
- Juul S, Nielsen EE, Feinberg J, Siddiqui F, Jørgensen CK, Barot E et al.Interventions for treatment of COVID-19: second edition of a living systematic review with meta-analyses and trial sequential analyses (The LIVING Project). medRxiv [Preprint] 2020.
Khan 2021
-
- Khan FA, Stewart I, Fabbri L, Moss S, Robinson K, Smyth AR, et al.Systematic review and meta-analysis of anakinra, sarilumab, siltuximab and tocilizumab for COVID-19. Thorax 2021;76(9):907-19. - PubMed
Kim 2020
Kirkham 2018
Kyriazopoulou 2021
-
- Kyriazopoulou E, Huet T, Cavalli G, Gori A, Kyprianou M, Pickkers P, et al, International Collaborative Group for Anakinra in COVID-19.Effect of anakinra on mortality in patients with COVID-19: a systematic review and patient-level meta-analysis. Lancet Rheumatology 2021;3(10):e690-7. - PMC - PubMed
La Rosée 2019
-
- La Rosée P, Horne A, Hines M, Bahr Greenwood T, Machowicz R, Berliner N, et al.Recommendations for the management of hemophagocytic lymphohistiocytosis in adults. Blood 2019;133(23):2465-77. - PubMed
Mavridis 2015
Mavridis 2018
Mullen 2020
-
- Mullen JL, Tsueng G, Abdel Latif A, Alkuzweny M, Cano M, Haag E et al.Outbreak.info. Available at: https://outbreak.info Accessed 24 January 2022.
Oikonomidi 2020
Ouzzani 2016
Pasin 2021
Peter 2021
Pierre 2021
Pile 2015
-
- Pile KD, Graham GG, Mahler SM.Interleukin 1 Inhibitors. In: Parnham M, editors(s). Encyclopedia of Inflammatory Diseases. Basel, Switzerland: Springer Basel, 2015:1-5.
Putman 2021
REACT 2021
Riley 2011
-
- Riley RD, Higgins JP, Deeks JJ.Interpretation of random effects meta-analyses. BMJ 2011;342:d549. - PubMed
Schünemann 2019
-
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al.Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Shakoory 2016
-
- Shakoory B, Carcillo JA, Chatham WW, Amdur RL, Zhao H, Dinarello CA, et al.Interleukin-1 receptor blockade is associated with reduced mortality in sepsis patients with features of macrophage activation syndrome: reanalysis of a prior phase III trial. Critical Care Medicine 2016;44(2):275-81. - PMC - PubMed
Siemieniuk 2020
Sims 2010
-
- Sims JE, Smith DE.The IL-1 family: regulators of immunity. Nature Reviews Immunology 2010;10(2):89-102. - PubMed
Somagutta 2021
Sterne 2019
-
- Sterne JA, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al.RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. - PubMed
Talaie 2020
Tierney 2007
Turner 2012
van de Veerdonk 2020
White 2008
-
- White IR, Higgins JP, Wood AM.Allowing for uncertainty due to missing data in meta-analysis--part 1: two-stage methods. Statistics in Medicine 2008;27(5):711-27. - PubMed
WHO 2020a
-
- World Health Organization.Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected: interim guidance; 12 January 2020. Available at: apps.who.int/iris/handle/10665/332299.
WHO 2020b
-
- World Health Organization.Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19). Available at https://www.who.int/publications/i/item/report-of-the-who-china-joint-mi....
WHO Working Group 2020
Worldometer 2020
-
- COVID Live - Coronavirus Statistics - Worldometer. Available at worldometers.info/coronavirus/ Accessed 12 November 2021.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Miscellaneous

