Poor reporting quality of observational clinical studies comparing treatments of COVID-19 - a retrospective cross-sectional study
- PMID: 35057739
- PMCID: PMC8771183
- DOI: 10.1186/s12874-021-01501-9
Poor reporting quality of observational clinical studies comparing treatments of COVID-19 - a retrospective cross-sectional study
Abstract
Background: During the COVID-19 pandemic, the scientific world is in urgent need for new evidence on the treatment of COVID patients. The reporting quality is crucial for transparent scientific publication. Concerns of data integrity, methodology and transparency were raised. Here, we assessed the adherence of observational studies comparing treatments of COVID 19 to the STROBE checklist in 2020.
Methods: Design: We performed a retrospective, cross-sectional study.
Setting: We conducted a systematic literature search in the Medline database. This study was performed at the RWTH Aachen University Hospital, Department of Anaesthesiology Participants: We extracted all observational studies on the treatment of COVID-19 patients from the year 2020.
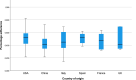
Main outcome measures: The adherence of each publication to the STROBE checklist items was analysed. The journals' impact factor (IF), the country of origin, the kind of investigated treatment and the month of publication were assessed.
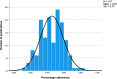
Results: We analysed 147 observational studies and found a mean adherence of 45.6% to the STROBE checklist items. The percentage adherence per publication correlated significantly with the journals' IF (point estimate for the difference between 1st and 4th quartile 11.07%, 95% CI 5.12 to 17.02, p < 0.001). U.S. American authors gained significantly higher adherence to the checklist than Chinese authors, mean difference 9.10% (SD 2.85%, p = 0.023).
Conclusions: We conclude a poor reporting quality of observational studies on the treatment of COVID-19 throughout the year 2020. A considerable improvement is mandatory.
Keywords: COVID-19; Observational studies; Reporting quality; STROBE statement.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Poor reporting quality of randomized controlled trials comparing treatments of COVID-19-A retrospective cross-sectional study on the first year of publications.PLoS One. 2023 Oct 16;18(10):e0292860. doi: 10.1371/journal.pone.0292860. eCollection 2023. PLoS One. 2023. PMID: 37844082 Free PMC article.
-
Publishing in pandemic times: A bibliometric analysis of early medical publications on Kawasaki-like disease (MIS-C, PIMS-TS) related to SARS-CoV-2.Arch Pediatr. 2021 Aug;28(6):464-469. doi: 10.1016/j.arcped.2021.05.002. Epub 2021 May 28. Arch Pediatr. 2021. PMID: 34140220
-
The reporting quality of observational studies relevant to the STROBE-nut statement in journals of nutrition.Asia Pac J Clin Nutr. 2021;30(1):174-183. doi: 10.6133/apjcn.202103_30(1).0020. Asia Pac J Clin Nutr. 2021. PMID: 33787053
-
Adherence of SARS-CoV-2 Seroepidemiologic Studies to the ROSES-S Reporting Guideline During the COVID-19 Pandemic.Influenza Other Respir Viruses. 2024 Jul;18(7):e13283. doi: 10.1111/irv.13283. Influenza Other Respir Viruses. 2024. PMID: 39053893 Free PMC article. Review.
-
Quality of Reporting and Study Design of CKD Cohort Studies Assessing Mortality in the Elderly Before and After STROBE: A Systematic Review.PLoS One. 2016 May 11;11(5):e0155078. doi: 10.1371/journal.pone.0155078. eCollection 2016. PLoS One. 2016. PMID: 27168187 Free PMC article. Review.
Cited by
-
Safety and preliminary efficacy of sequential multiple ascending doses of solnatide to treat pulmonary permeability edema in patients with moderate to severe ARDS in a randomized, placebo-controlled, double-blind trial: preliminary evaluation of safety and feasibility in light of the COVID-19 pandemic.Trials. 2022 Apr 4;23(1):252. doi: 10.1186/s13063-022-06182-3. Trials. 2022. PMID: 35379296 Free PMC article. Clinical Trial.
-
A Systematic Review to Inform the Development of a Reporting Guideline for Concept Mapping Research.Methods Protoc. 2023 Oct 17;6(5):101. doi: 10.3390/mps6050101. Methods Protoc. 2023. PMID: 37888033 Free PMC article. Review.
-
Poor reporting quality of randomized controlled trials comparing treatments of COVID-19-A retrospective cross-sectional study on the first year of publications.PLoS One. 2023 Oct 16;18(10):e0292860. doi: 10.1371/journal.pone.0292860. eCollection 2023. PLoS One. 2023. PMID: 37844082 Free PMC article.
-
Visualizing the target estimand in comparative effectiveness studies with multiple treatments.J Comp Eff Res. 2024 Feb;13(2):e230089. doi: 10.57264/cer-2023-0089. Epub 2024 Jan 23. J Comp Eff Res. 2024. PMID: 38261336 Free PMC article.
-
Reporting of Observational Studies Explicitly Aiming to Emulate Randomized Trials: A Systematic Review.JAMA Netw Open. 2023 Sep 5;6(9):e2336023. doi: 10.1001/jamanetworkopen.2023.36023. JAMA Netw Open. 2023. PMID: 37755828 Free PMC article.
References
-
- von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, Initiative S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Epidemiology. 2007;18(6):800–804. doi: 10.1097/EDE.0b013e3181577654. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

