Targeting Histone Deacetylases: Opportunities for Cancer Treatment and Chemoprevention
- PMID: 35057104
- PMCID: PMC8778744
- DOI: 10.3390/pharmaceutics14010209
Targeting Histone Deacetylases: Opportunities for Cancer Treatment and Chemoprevention
Abstract
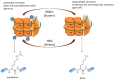
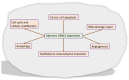
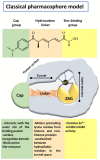
The dysregulation of gene expression is a critical event involved in all steps of tumorigenesis. Aberrant histone and non-histone acetylation modifications of gene expression due to the abnormal activation of histone deacetylases (HDAC) have been reported in hematologic and solid types of cancer. In this sense, the cancer-associated epigenetic alterations are promising targets for anticancer therapy and chemoprevention. HDAC inhibitors (HDACi) induce histone hyperacetylation within target proteins, altering cell cycle and proliferation, cell differentiation, and the regulation of cell death programs. Over the last three decades, an increasing number of synthetic and naturally derived compounds, such as dietary-derived products, have been demonstrated to act as HDACi and have provided biological and molecular insights with regard to the role of HDAC in cancer. The first part of this review is focused on the biological roles of the Zinc-dependent HDAC family in malignant diseases. Accordingly, the small-molecules and natural products such as HDACi are described in terms of cancer therapy and chemoprevention. Furthermore, structural considerations are included to improve the HDACi selectivity and combinatory potential with other specific targeting agents in bifunctional inhibitors and proteolysis targeting chimeras. Additionally, clinical trials that combine HDACi with current therapies are discussed, which may open new avenues in terms of the feasibility of HDACi's future clinical applications in precision cancer therapies.
Keywords: HDAC inhibitors; PROTAC; bifunctional inhibitors; cancer; chemoprevention; clinical trials; dietary-derived inhibitors; epigenetic; histone deacetylases.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Histone Deacetylase Inhibition in Non-small Cell Lung Cancer: Hype or Hope?Front Cell Dev Biol. 2020 Oct 9;8:582370. doi: 10.3389/fcell.2020.582370. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33163495 Free PMC article. Review.
-
The Roles of Histone Deacetylases and Their Inhibitors in Cancer Therapy.Front Cell Dev Biol. 2020 Sep 29;8:576946. doi: 10.3389/fcell.2020.576946. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33117804 Free PMC article. Review.
-
Prospects: histone deacetylase inhibitors.J Cell Biochem. 2005 Oct 1;96(2):293-304. doi: 10.1002/jcb.20532. J Cell Biochem. 2005. PMID: 16088937 Review.
-
Roles of Histone Deacetylases and Inhibitors in Anticancer Therapy.Cancers (Basel). 2020 Jun 23;12(6):1664. doi: 10.3390/cancers12061664. Cancers (Basel). 2020. PMID: 32585896 Free PMC article. Review.
-
Impact of HDAC Inhibitors on Protein Quality Control Systems: Consequences for Precision Medicine in Malignant Disease.Front Cell Dev Biol. 2020 Jun 3;8:425. doi: 10.3389/fcell.2020.00425. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32582706 Free PMC article. Review.
Cited by
-
Comprehensive analyses reveal the role of histone deacetylase genes in prognosis and immune response in low-grade glioma.PLoS One. 2022 Oct 13;17(10):e0276120. doi: 10.1371/journal.pone.0276120. eCollection 2022. PLoS One. 2022. PMID: 36227941 Free PMC article.
-
Insights Into the Properties, Biological Functions, and Regulation of USP21.Front Pharmacol. 2022 Jun 30;13:944089. doi: 10.3389/fphar.2022.944089. eCollection 2022. Front Pharmacol. 2022. PMID: 35846989 Free PMC article. Review.
-
Recent Advances in the Applications of Small Molecules in the Treatment of Multiple Myeloma.Int J Mol Sci. 2023 Jan 31;24(3):2645. doi: 10.3390/ijms24032645. Int J Mol Sci. 2023. PMID: 36768967 Free PMC article. Review.
-
Current and Future Cancer Chemoprevention Strategies.Pharmaceutics. 2023 May 19;15(5):1543. doi: 10.3390/pharmaceutics15051543. Pharmaceutics. 2023. PMID: 37242785 Free PMC article.
-
Potential of Synthetic and Natural Compounds as Novel Histone Deacetylase Inhibitors for the Treatment of Hematological Malignancies.Cancers (Basel). 2023 May 17;15(10):2808. doi: 10.3390/cancers15102808. Cancers (Basel). 2023. PMID: 37345145 Free PMC article. Review.
References
-
- Fraga M.F., Ballestar E., Villar-Garea A., Boix-Chornet M., Espada J., Schotta G., Bonaldi T., Haydon C., Ropero S., Petrie K., et al. Loss of acetylation at Lys16 and trimethylation at Lys20 of histone H4 is a common hallmark of human cancer. Nat. Genet. 2005;37:391–400. doi: 10.1038/ng1531. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

