Renal mTORC1 activation is associated with disease activity and prognosis in lupus nephritis
- PMID: 35040950
- PMCID: PMC9608003
- DOI: 10.1093/rheumatology/keac037
Renal mTORC1 activation is associated with disease activity and prognosis in lupus nephritis
Abstract
Objective: This study was initiated to evaluate mammalian target of rapamycin (mTOR) activation in renal tissue of LN patients.
Methods: This retrospective study included 187 LN patients, 20 diabetic nephropathy (DN) patients, 10 minimal change disease (MCD) patients and 10 normal controls (NCs). Seven of 187 LN patients had repeated renal biopsies. mTORC1/2 activation was evaluated by immunohistochemistry and multiplexed immunofluorescence. The association of mTORC1/2 activation with the clinicopathologic indices and prognostic outcomes was analysed among 187 LN patients. Proteomics was performed in renal biopsies of 20 LN patients. Proteomics was employed to comprehensively evaluate the impact of mTOR activation on intrarenal gene expression.
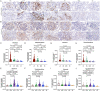
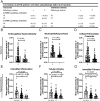
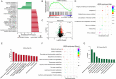
Results: mTORC1/2 was significantly activated in podocytes, mesangial cells, endothelial cells and tubular epithelial cells of LN patients as compared with those with MCD or NC. The glomerular mTORC1 activation was higher in LN patients compared with DN patients. mTORC1, but not mTORC2, activation strongly correlated with serum albumin, complement C3, proteinuria and the following pathological biomarkers of LN: crescent formation, interstitial inflammation and fibrosis. Moreover, mTORC1 activation was identified as a prognostic marker in LN patients. Bioinformatic analyses of proteomics and immunohistochemical data unveiled increased complement activation, antigen presentation and phagocytosis in LN patients with mTORC1 activation.
Conclusion: Renal mTORC1 activation could be a biomarker to reveal disease activity and predict clinical prognosis in LN patients.
Keywords: bioinformatics; lupus nephritis; mTOR; proteomics; rapamycin.
© The Author(s) 2022. Published by Oxford University Press on behalf of the British Society for Rheumatology. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures

Similar articles
-
Glomerular mTORC1 activation was associated with podocytes to endothelial cells communication in lupus nephritis.Lupus Sci Med. 2023 May;10(1):e000896. doi: 10.1136/lupus-2023-000896. Lupus Sci Med. 2023. PMID: 37147021 Free PMC article.
-
mTORC1 activation induced proximal tubular damage via the pentose phosphate pathway in lupus nephritis.Free Radic Biol Med. 2022 Aug 20;189:91-101. doi: 10.1016/j.freeradbiomed.2022.07.010. Epub 2022 Jul 19. Free Radic Biol Med. 2022. PMID: 35863688
-
Association between glomerular mTORC1 activation and crescents formation in lupus nephritis patients.Clin Immunol. 2023 Apr;249:109288. doi: 10.1016/j.clim.2023.109288. Epub 2023 Mar 11. Clin Immunol. 2023. PMID: 36907538
-
Cell type-specific mechanistic target of rapamycin-dependent distortion of autophagy pathways in lupus nephritis.Transl Res. 2022 Jul;245:55-81. doi: 10.1016/j.trsl.2022.03.004. Epub 2022 Mar 12. Transl Res. 2022. PMID: 35288362 Free PMC article. Review.
-
The Emerging Role of Renal Tubular Epithelial Cells in the Immunological Pathophysiology of Lupus Nephritis.Front Immunol. 2020 Sep 23;11:578952. doi: 10.3389/fimmu.2020.578952. eCollection 2020. Front Immunol. 2020. PMID: 33072122 Free PMC article. Review.
Cited by
-
Safety and efficacy of the SGLT2 inhibitor dapagliflozin in patients with systemic lupus erythematosus: a phase I/II trial.RMD Open. 2022 Oct;8(2):e002686. doi: 10.1136/rmdopen-2022-002686. RMD Open. 2022. PMID: 36288823 Free PMC article. Clinical Trial.
-
Advanced methods and novel biomarkers in autoimmune diseases ‑ a review of the recent years progress in systemic lupus erythematosus.Front Med (Lausanne). 2023 Jun 23;10:1183535. doi: 10.3389/fmed.2023.1183535. eCollection 2023. Front Med (Lausanne). 2023. PMID: 37425332 Free PMC article. Review.
-
Principles behind SLE treatment with N-acetylcysteine.Immunometabolism (Cobham). 2022 Oct 25;4(4):e00010. doi: 10.1097/IN9.0000000000000010. eCollection 2022 Oct. Immunometabolism (Cobham). 2022. PMID: 36312742 Free PMC article. Review.
-
Rictor/mTORC2 signalling contributes to renal vascular endothelial-to-mesenchymal transition and renal allograft interstitial fibrosis by regulating BNIP3-mediated mitophagy.Clin Transl Med. 2024 May;14(5):e1686. doi: 10.1002/ctm2.1686. Clin Transl Med. 2024. PMID: 38769658 Free PMC article.
-
Lupus Nephritis: Current Perspectives and Moving Forward.J Inflamm Res. 2022 Dec 2;15:6533-6552. doi: 10.2147/JIR.S363722. eCollection 2022. J Inflamm Res. 2022. PMID: 36483271 Free PMC article. Review.
References
-
- Yu F, Haas M, Glassock R, Zhao MH.. Redefining lupus nephritis: clinical implications of pathophysiologic subtypes. Nat Rev Nephrol 2017;13:483–95. - PubMed
-
- Cameron JS. Lupus nephritis. J Am Soc Nephrol 1999;10:413–24. - PubMed
-
- D’Cruz DP, Khamashta MA, Hughes GR.. Systemic lupus erythematosus. Lancet 2007;369:587–96. - PubMed
-
- Ginzler EM, Dooley MA, Aranow C. et al. Mycophenolate mofetil or intravenous cyclophosphamide for lupus nephritis. N Engl J Med 2005;353:2219–28. - PubMed
-
- Anders HJ, Saxena R, Zhao MH. et al. Lupus nephritis. Nat Rev Dis Primers 2020;6:7. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

