Regulation of Myostatin on the Growth and Development of Skeletal Muscle
- PMID: 35004684
- PMCID: PMC8740192
- DOI: 10.3389/fcell.2021.785712
Regulation of Myostatin on the Growth and Development of Skeletal Muscle
Abstract
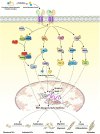
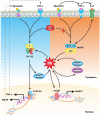
Myostatin (MSTN), a member of the transforming growth factor-β superfamily, can negatively regulate the growth and development of skeletal muscle by autocrine or paracrine signaling. Mutation of the myostatin gene under artificial or natural conditions can lead to a significant increase in muscle quality and produce a double-muscle phenotype. Here, we review the similarities and differences between myostatin and other members of the transforming growth factor-β superfamily and the mechanisms of myostatin self-regulation. In addition, we focus extensively on the regulation of myostatin functions involved in myogenic differentiation, myofiber type conversion, and skeletal muscle protein synthesis and degradation. Also, we summarize the induction of reactive oxygen species generation and oxidative stress by myostatin in skeletal muscle. This review of recent insights into the function of myostatin will provide reference information for future studies of myostatin-regulated skeletal muscle formation and may have relevance to agricultural fields of study.
Keywords: degradation; myogenesis; myostatin; protein synthesis; skeletal muscle development.
Copyright © 2021 Chen, Zhao, Zhao, Deng and Yu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Insulin-like growth factor-1 suppresses the Myostatin signaling pathway during myogenic differentiation.Biochem Biophys Res Commun. 2015 Aug 21;464(2):596-602. doi: 10.1016/j.bbrc.2015.07.018. Epub 2015 Jul 4. Biochem Biophys Res Commun. 2015. PMID: 26151859
-
Myostatin is an inhibitor of myogenic differentiation.Am J Physiol Cell Physiol. 2002 May;282(5):C993-9. doi: 10.1152/ajpcell.00372.2001. Am J Physiol Cell Physiol. 2002. PMID: 11940514
-
Modulation of reactive oxygen species in skeletal muscle by myostatin is mediated through NF-κB.Aging Cell. 2011 Dec;10(6):931-48. doi: 10.1111/j.1474-9726.2011.00734.x. Epub 2011 Aug 16. Aging Cell. 2011. PMID: 21771249 Free PMC article.
-
Myostatin regulation of muscle development: molecular basis, natural mutations, physiopathological aspects.Exp Cell Res. 2006 Aug 1;312(13):2401-14. doi: 10.1016/j.yexcr.2006.04.012. Epub 2006 May 3. Exp Cell Res. 2006. PMID: 16793037 Review.
-
Transforming growth factor-beta and myostatin signaling in skeletal muscle.J Appl Physiol (1985). 2008 Mar;104(3):579-87. doi: 10.1152/japplphysiol.01091.2007. Epub 2007 Nov 21. J Appl Physiol (1985). 2008. PMID: 18032576 Review.
Cited by
-
New Trends to Treat Muscular Atrophy: A Systematic Review of Epicatechin.Nutrients. 2024 Jan 22;16(2):326. doi: 10.3390/nu16020326. Nutrients. 2024. PMID: 38276564 Free PMC article. Review.
-
Fecal transplant from myostatin deletion pigs positively impacts the gut-muscle axis.Elife. 2023 Apr 11;12:e81858. doi: 10.7554/eLife.81858. Elife. 2023. PMID: 37039469 Free PMC article.
-
Restorative effects of (+)-epicatechin in a rodent model of aging induced muscle atrophy: underlying mechanisms.Food Funct. 2024 Apr 2;15(7):3669-3679. doi: 10.1039/d3fo04004f. Food Funct. 2024. PMID: 38487922
-
Effects of Creatine Supplementation on the Myostatin Pathway and Myosin Heavy Chain Isoforms in Different Skeletal Muscles of Resistance-Trained Rats.Nutrients. 2023 May 8;15(9):2224. doi: 10.3390/nu15092224. Nutrients. 2023. PMID: 37432386 Free PMC article.
-
Exercise Induced-Cytokines Response in Marathon Runners: Role of ACE I/D and BDKRB2 +9/-9 Polymorphisms.Front Physiol. 2022 Sep 2;13:919544. doi: 10.3389/fphys.2022.919544. eCollection 2022. Front Physiol. 2022. PMID: 36117688 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

