LRRK2 and idiopathic Parkinson's disease
- PMID: 34991886
- PMCID: PMC8854345
- DOI: 10.1016/j.tins.2021.12.002
LRRK2 and idiopathic Parkinson's disease
Abstract
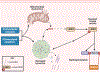
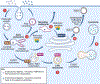
The etiology of idiopathic Parkinson's disease (iPD) is multifactorial, and both genetics and environmental exposures are risk factors. While mutations in leucine-rich repeat kinase-2 (LRRK2) that are associated with increased kinase activity are the most common cause of autosomal dominant PD, the role of LRRK2 in iPD, independent of mutations, remains uncertain. In this review, we discuss how the architecture of LRRK2 influences kinase activation and how enhanced LRRK2 substrate phosphorylation might contribute to pathogenesis. We describe how oxidative stress and endolysosomal dysfunction, both of which occur in iPD, can activate non-mutated LRRK2 to a similar degree as pathogenic mutations. Similarly, environmental toxicants that are linked epidemiologically to iPD risk can also activate LRRK2. In aggregate, current evidence suggests an important role for LRRK2 in iPD.
Keywords: autophagy–lysosomal pathway; endogenous protein expression; environmental toxicants; kinase; oxidative stress.
Copyright © 2021 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests None declared by authors.
Figures
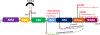

Similar articles
-
LRRK2 activation in idiopathic Parkinson's disease.Sci Transl Med. 2018 Jul 25;10(451):eaar5429. doi: 10.1126/scitranslmed.aar5429. Sci Transl Med. 2018. PMID: 30045977 Free PMC article.
-
LRRK2 and the Endolysosomal System in Parkinson's Disease.J Parkinsons Dis. 2020;10(4):1271-1291. doi: 10.3233/JPD-202138. J Parkinsons Dis. 2020. PMID: 33044192 Free PMC article. Review.
-
LRRK2, GBA and their interaction in the regulation of autophagy: implications on therapeutics in Parkinson's disease.Transl Neurodegener. 2022 Jan 31;11(1):5. doi: 10.1186/s40035-022-00281-6. Transl Neurodegener. 2022. PMID: 35101134 Free PMC article. Review.
-
Advances in elucidating the function of leucine-rich repeat protein kinase-2 in normal cells and Parkinson's disease.Curr Opin Cell Biol. 2020 Apr;63:102-113. doi: 10.1016/j.ceb.2020.01.001. Epub 2020 Feb 7. Curr Opin Cell Biol. 2020. PMID: 32036294 Free PMC article. Review.
-
The G2019S variant of leucine-rich repeat kinase 2 (LRRK2) alters endolysosomal trafficking by impairing the function of the GTPase RAB8A.J Biol Chem. 2019 Mar 29;294(13):4738-4758. doi: 10.1074/jbc.RA118.005008. Epub 2019 Feb 1. J Biol Chem. 2019. PMID: 30709905 Free PMC article.
Cited by
-
An unbiased, automated platform for scoring dopaminergic neurodegeneration in C. elegans.bioRxiv [Preprint]. 2023 Feb 3:2023.02.02.526781. doi: 10.1101/2023.02.02.526781. bioRxiv. 2023. Update in: PLoS One. 2023 Jul 7;18(7):e0281797. doi: 10.1371/journal.pone.0281797 PMID: 36778421 Free PMC article. Updated. Preprint.
-
NADPH oxidase 2 activity in Parkinson's disease.Neurobiol Dis. 2022 Aug;170:105754. doi: 10.1016/j.nbd.2022.105754. Epub 2022 May 13. Neurobiol Dis. 2022. PMID: 35577065 Free PMC article.
-
Peptide-based approaches to directly target alpha-synuclein in Parkinson's disease.Mol Neurodegener. 2023 Nov 9;18(1):80. doi: 10.1186/s13024-023-00675-8. Mol Neurodegener. 2023. PMID: 37940962 Free PMC article. Review.
-
Exploitation of porphyrin-based titanium-rich porous organic polymers for targeted phosphopeptide enrichment from the serum of colorectal cancer individuals.Mikrochim Acta. 2024 Jul 27;191(8):487. doi: 10.1007/s00604-024-06561-4. Mikrochim Acta. 2024. PMID: 39060411
-
Loss of mitochondrial Ca2+ response and CaMKII/ERK activation by LRRK2R1441G mutation correlate with impaired depolarization-induced mitophagy.Cell Commun Signal. 2024 Oct 10;22(1):485. doi: 10.1186/s12964-024-01844-y. Cell Commun Signal. 2024. PMID: 39390438 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

