Structural basis of HLX10 PD-1 receptor recognition, a promising anti-PD-1 antibody clinical candidate for cancer immunotherapy
- PMID: 34972111
- PMCID: PMC8719770
- DOI: 10.1371/journal.pone.0257972
Structural basis of HLX10 PD-1 receptor recognition, a promising anti-PD-1 antibody clinical candidate for cancer immunotherapy
Abstract
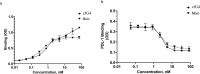
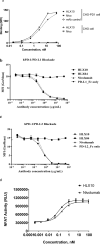
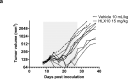
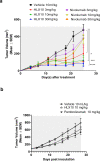
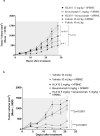
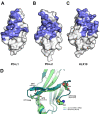
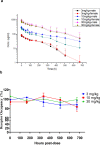
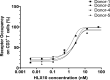
Cancer immunotherapies, such as checkpoint blockade of programmed cell death protein-1 (PD-1), represents a breakthrough in cancer treatment, resulting in unprecedented results in terms of overall and progression-free survival. Discovery and development of novel anti PD-1 inhibitors remains a field of intense investigation, where novel monoclonal antibodies (mAbs) and novel antibody formats (e.g., novel isotype, bispecific mAb and low-molecular-weight compounds) are major source of future therapeutic candidates. HLX10, a fully humanized IgG4 monoclonal antibody against PD-1 receptor, increased functional activities of human T-cells and showed in vitro, and anti-tumor activity in several tumor models. The combined inhibition of PD-1/PDL-1 and angiogenesis pathways using anti-VEGF antibody may enhance a sustained suppression of cancer-related angiogenesis and tumor elimination. To elucidate HLX10's mode of action, we solved the structure of HLX10 in complex with PD-1 receptor. Detailed epitope analysis showed that HLX10 has a unique mode of recognition compared to the clinically approved PD1 antibodies Pembrolizumab and Nivolumab. Notably, HLX10's epitope was closer to Pembrolizumab's epitope than Nivolumab's epitope. However, HLX10 and Pembrolizumab showed an opposite heavy chain (HC) and light chain (LC) usage, which recognizes several overlapping amino acid residues on PD-1. We compared HLX10 to Nivolumab and Pembrolizumab and it showed similar or better bioactivity in vitro and in vivo, providing a rationale for clinical evaluation in cancer immunotherapy.
Conflict of interest statement
The study is about HLX10; a clinical lead currently in development in China and US. Henlius filled patent in US (US2021277122A1). There are no additional patents, products in development or marketed products associated with this research to declare. This work does not alter PLOS’s adherence policies on sharing data and materials.
Figures


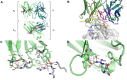

Similar articles
-
Sym021, a promising anti-PD1 clinical candidate antibody derived from a new chicken antibody discovery platform.MAbs. 2019 May/Jun;11(4):666-680. doi: 10.1080/19420862.2019.1596514. Epub 2019 May 3. MAbs. 2019. PMID: 31046547 Free PMC article. Clinical Trial.
-
Structural basis of checkpoint blockade by monoclonal antibodies in cancer immunotherapy.Nat Commun. 2016 Oct 31;7:13354. doi: 10.1038/ncomms13354. Nat Commun. 2016. PMID: 27796306 Free PMC article.
-
Durable blockade of PD-1 signaling links preclinical efficacy of sintilimab to its clinical benefit.MAbs. 2019 Nov-Dec;11(8):1443-1451. doi: 10.1080/19420862.2019.1654303. Epub 2019 Sep 3. MAbs. 2019. PMID: 31402780 Free PMC article.
-
Cervical cancer - State of the science: From angiogenesis blockade to checkpoint inhibition.Gynecol Oncol. 2018 Mar;148(3):609-621. doi: 10.1016/j.ygyno.2018.01.009. Epub 2018 Feb 3. Gynecol Oncol. 2018. PMID: 29666026 Free PMC article. Review.
-
Programmed death-1 & its ligands: promising targets for cancer immunotherapy.Immunotherapy. 2015;7(7):777-92. doi: 10.2217/imt.15.49. Epub 2015 Aug 7. Immunotherapy. 2015. PMID: 26250412 Review.
Cited by
-
Effect of First-Line Serplulimab vs Placebo Added to Chemotherapy on Survival in Patients With Extensive-Stage Small Cell Lung Cancer: The ASTRUM-005 Randomized Clinical Trial.JAMA. 2022 Sep 27;328(12):1223-1232. doi: 10.1001/jama.2022.16464. JAMA. 2022. PMID: 36166026 Free PMC article. Clinical Trial.
-
Advances in immune checkpoint inhibitors therapy for small cell lung cancer.Cancer Med. 2023 May;12(10):11097-11106. doi: 10.1002/cam4.5659. Epub 2023 Mar 7. Cancer Med. 2023. PMID: 36880420 Free PMC article. Review.
-
Serplulimab combined with gemcitabine, nab-paclitaxel and stereotactic body radiotherapy as the first-line treatment for patients with metastatic pancreatic adenocarcinoma in China: a multicentre, single-arm, phase II trial (ICSBR) protocol.BMJ Open. 2024 Jul 16;14(7):e084274. doi: 10.1136/bmjopen-2024-084274. BMJ Open. 2024. PMID: 39013651 Free PMC article.
-
Efficacy and safety of different immunotherapies combined with chemotherapy as first-line therapy in patients with small cell lung cancer: a network meta-analysis.Front Immunol. 2024 Apr 17;15:1362537. doi: 10.3389/fimmu.2024.1362537. eCollection 2024. Front Immunol. 2024. PMID: 38694505 Free PMC article.
-
The Optimal First-Line Therapy for Extensive-Stage Small-Cell Lung Cancer Based on Liver Metastasis Status: A Network Meta-Analysis and Systematic Review.Cancer Med. 2024 Sep;13(18):e70256. doi: 10.1002/cam4.70256. Cancer Med. 2024. PMID: 39358989 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

