An alternatively spliced STING isoform localizes in the cytoplasmic membrane and directly senses extracellular cGAMP
- PMID: 34905508
- PMCID: PMC8803335
- DOI: 10.1172/JCI144339
An alternatively spliced STING isoform localizes in the cytoplasmic membrane and directly senses extracellular cGAMP
Abstract
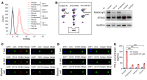
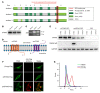
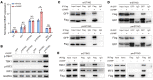
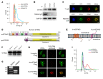
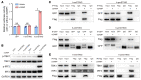
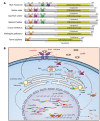
It has been revealed that 2'3'-cyclic-GMP-AMP (cGAMP), a second messenger that activates the antiviral stimulator of IFN genes (STING), elicits an antitumoral immune response. Since cGAMP cannot cross the cell membrane, it is not clear how intracellular STING has been activated by extracellular cGAMP until SLC19A1 was identified as an importer to transport extracellular cGAMP into the cytosol. However, SLC19A1-deficient cells also sense extracellular cGAMP, suggesting the presence of mechanisms other than the facilitating transporters for STING sensing extracellular cGAMP. Here, using immunoprecipitation, immunofluorescence, and flow cytometry, we identified an alternatively spliced STING isoform, plasmatic membrane STING (pmSTING), that localized in the plasma membrane with its C-terminus outside the cell, due to a lack of 1 transmembrane domain in its N-terminus compared with canonical STING. Further studies showed that extracellular cGAMP not only promoted the dimerization of pmSTING and interaction of pmSTING with TANK-binding kinase 1 (TBK1) and IFN regulatory factor 3 (IRF3), but also enhanced the phosphorylation of TBK1 and IRF3 and the production of IFN in pmSTING-transfected cells. Additionally, we also identified similar pmSTING isoforms in other species including human. This study suggests a conserved role for pmSTING in sensing extracellular cGAMP and provides insight into the role of cGAMP as an immunotransmitter.
Keywords: Cancer immunotherapy; Cellular immune response; Immunology; Innate immunity; Oncology.
Figures

Similar articles
-
SLC19A1 Is an Importer of the Immunotransmitter cGAMP.Mol Cell. 2019 Jul 25;75(2):372-381.e5. doi: 10.1016/j.molcel.2019.05.006. Epub 2019 May 21. Mol Cell. 2019. PMID: 31126740 Free PMC article.
-
Structural basis of STING binding with and phosphorylation by TBK1.Nature. 2019 Mar;567(7748):394-398. doi: 10.1038/s41586-019-1000-2. Epub 2019 Mar 6. Nature. 2019. PMID: 30842653 Free PMC article.
-
A novel transcript isoform of STING that sequesters cGAMP and dominantly inhibits innate nucleic acid sensing.Nucleic Acids Res. 2018 May 4;46(8):4054-4071. doi: 10.1093/nar/gky186. Nucleic Acids Res. 2018. PMID: 29547894 Free PMC article.
-
Second messenger 2'3'-cyclic GMP-AMP (2'3'-cGAMP): the cell autonomous and non-autonomous roles in cancer progression.Acta Pharmacol Sin. 2024 May;45(5):890-899. doi: 10.1038/s41401-023-01210-7. Epub 2024 Jan 4. Acta Pharmacol Sin. 2024. PMID: 38177693 Review.
-
cGAS/cGAMP/STING signal propagation in the tumor microenvironment: Key role for myeloid cells in antitumor immunity.Radiother Oncol. 2022 Sep;174:158-167. doi: 10.1016/j.radonc.2022.07.014. Epub 2022 Jul 20. Radiother Oncol. 2022. PMID: 35870728 Review.
Cited by
-
Understanding and therapeutically exploiting cGAS/STING signaling in glioblastoma.J Clin Invest. 2024 Jan 16;134(2):e163452. doi: 10.1172/JCI163452. J Clin Invest. 2024. PMID: 38226619 Free PMC article. Review.
-
cGAS-STING signaling pathway in intestinal homeostasis and diseases.Front Immunol. 2023 Sep 14;14:1239142. doi: 10.3389/fimmu.2023.1239142. eCollection 2023. Front Immunol. 2023. PMID: 37781354 Free PMC article. Review.
-
Signal strength of STING activation determines cytokine plasticity and cell death in human monocytes.Sci Rep. 2022 Oct 24;12(1):17827. doi: 10.1038/s41598-022-20519-7. Sci Rep. 2022. PMID: 36280676 Free PMC article.
-
Time-course RNA-Seq profiling reveals isoform-level gene expression dynamics of the cGAS-STING pathway.Comput Struct Biotechnol J. 2022;20:6490-6500. doi: 10.1016/j.csbj.2022.11.044. Epub 2022 Nov 24. Comput Struct Biotechnol J. 2022. PMID: 36448027 Free PMC article.
-
Protocol for the identification and expression analysis of a cytoplasmic membrane-localized protein STING.STAR Protoc. 2023 Mar 20;4(2):102172. doi: 10.1016/j.xpro.2023.102172. Online ahead of print. STAR Protoc. 2023. PMID: 36943863 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

