Community-Based Testing Sites for SARS-CoV-2 - United States, March 2020-November 2021
- PMID: 34882655
- PMCID: PMC8659188
- DOI: 10.15585/mmwr.mm7049a3
Community-Based Testing Sites for SARS-CoV-2 - United States, March 2020-November 2021
Abstract
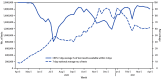
Immediately following the March 13, 2020 declaration of COVID-19 as a national emergency (1), the U.S. government began implementing national testing programs for epidemiologic surveillance, monitoring of frontline workers and populations at higher risk for acquiring COVID-19, and identifying and allocating limited testing resources. Effective testing supports identification of COVID-19 cases; facilitates isolation, quarantine, and timely treatment measures that limit the spread of SARS-CoV-2 (the virus that causes COVID-19); and guides public health officials about the incidence of COVID-19 in a community. A White House Joint Task Force, co-led by the Department of Health and Human Services (HHS) and the Federal Emergency Management Agency (FEMA), created the Community-Based Testing Sites (CBTS) program working with state and local partners (2). This report describes the timeline, services delivered, and scope of the CBTS program. During March 19, 2020-April 11, 2021, the CBTS program conducted 11,661,923 SARS-CoV-2 tests at 8,319 locations across the United States and its territories, including 402,223 (3.5%) administered through Drive-Through Testing, 10,129,142 (86.9%) through Pharmacies+ Testing, and 1,130,558 (9.7%) through Surge Testing programs. Tests administered through the CBTS program yielded 1,176,959 (10.1%) positive results for SARS-CoV-2. Among tested persons with available race data,* positive test results were highest among American Indian or Alaska Native (14.1%) and Black persons (10.4%) and lowest among White persons (9.9%), Asian persons (7.3%), and Native Hawaiian or Other Pacific Islanders (6.4%). Among persons with reported ethnicity, 25.3% were Hispanic, 15.9% of whom received a positive test result. Overall, 82.0% of test results were returned within 2 days, but the percentage of test results returned within 2 days was as low as 40.7% in July 2020 and 59.3% in December 2020 during peak testing periods. Strong partnerships enabled a rapid coordinated response to establish the federally supported CBTS program to improve access to no-charge diagnostic testing, including for frontline workers, symptomatic persons and close contacts, and persons living in high-prevalence areas. In April 2021, the CBTS Pharmacies+ Testing and Surge Testing programs were expanded into the Increasing Community Access to Testing (ICATT) program. As of November 12, 2021, the CBTS and ICATT programs conducted approximately 26.6 million tests with approximately 10,000 active testing sites. Although the CBTS program represented a relatively small portion of overall U.S. SARS-CoV-2 testing, with its successful partnerships and adaptability, the CBTS program serves as a model to guide current community-based screening, surveillance, and disease control programs, and responses to future public health emergencies.
Conflict of interest statement
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. Mark F. Miller, Min Shi, Alison Motsinger-Reif, and Clarice R. Weinberg report receipt of intramural research funds from the National Institute of Environmental Health Sciences, Nationl Institutes of Health. No other potential conflicts of interest were disclosed.
Figures
Similar articles
-
Health Center Testing for SARS-CoV-2 During the COVID-19 Pandemic - United States, June 5-October 2, 2020.MMWR Morb Mortal Wkly Rep. 2020 Dec 18;69(50):1895-1901. doi: 10.15585/mmwr.mm6950a3. MMWR Morb Mortal Wkly Rep. 2020. PMID: 33332299 Free PMC article.
-
Assessment of Day-7 Postexposure Testing of Asymptomatic Contacts of COVID-19 Patients to Evaluate Early Release from Quarantine - Vermont, May-November 2020.MMWR Morb Mortal Wkly Rep. 2021 Jan 8;70(1):12-13. doi: 10.15585/mmwr.mm7001a3. MMWR Morb Mortal Wkly Rep. 2021. PMID: 33411700 Free PMC article.
-
Community-Based Testing for SARS-CoV-2 - Chicago, Illinois, May-November 2020.MMWR Morb Mortal Wkly Rep. 2021 May 14;70(19):707-711. doi: 10.15585/mmwr.mm7019a4. MMWR Morb Mortal Wkly Rep. 2021. PMID: 33983914 Free PMC article.
-
The requirements of nucleic acid test for COVID-19 during public health emergency: Current regulatory in Taiwan, Singapore, and the United States.J Chin Med Assoc. 2022 Nov 1;85(11):1038-1043. doi: 10.1097/JCMA.0000000000000804. Epub 2022 Nov 2. J Chin Med Assoc. 2022. PMID: 36343271 Review.
-
Standardizing, harmonizing, and protecting data collection to broaden the impact of COVID-19 research: the rapid acceleration of diagnostics-underserved populations (RADx-UP) initiative.J Am Med Inform Assoc. 2022 Aug 16;29(9):1480-1488. doi: 10.1093/jamia/ocac097. J Am Med Inform Assoc. 2022. PMID: 35678579 Free PMC article. Review.
Cited by
-
Increases in CLIA-Waived Testing Sites Since the Start of the COVID-19 Pandemic.Lab Med. 2023 Mar 7;54(2):126-129. doi: 10.1093/labmed/lmac154. Lab Med. 2023. PMID: 36638188 Free PMC article.
-
Association Between 3 Doses of mRNA COVID-19 Vaccine and Symptomatic Infection Caused by the SARS-CoV-2 Omicron and Delta Variants.JAMA. 2022 Feb 15;327(7):639-651. doi: 10.1001/jama.2022.0470. JAMA. 2022. PMID: 35060999 Free PMC article.
-
Association of Prior BNT162b2 COVID-19 Vaccination With Symptomatic SARS-CoV-2 Infection in Children and Adolescents During Omicron Predominance.JAMA. 2022 Jun 14;327(22):2210-2219. doi: 10.1001/jama.2022.7493. JAMA. 2022. PMID: 35560036 Free PMC article.
-
Development, testing and validation of a SARS-CoV-2 multiplex panel for detection of the five major variants of concern on a portable PCR platform.Front Public Health. 2022 Dec 15;10:1042647. doi: 10.3389/fpubh.2022.1042647. eCollection 2022. Front Public Health. 2022. PMID: 36590003 Free PMC article.
-
Reaching Structurally Vulnerable Populations Using Low-Barrier COVID-19 Testing Clinics Co-Created with Community-Based Organizations.J Gen Intern Med. 2024 Nov;39(15):3018-3027. doi: 10.1007/s11606-024-08889-2. Epub 2024 Jul 17. J Gen Intern Med. 2024. PMID: 39020226
References
-
- Office of the President of the United States. Proclamation on declaring a national emergency concerning the novel coronavirus disease (COVID-19) outbreak, March 13, 2020. Washington, DC: Office of the President of the United States; 2020. https://trumpwhitehouse.archives.gov/presidential-actions/proclamation-d...
-
- Office of the President of the United States. Remarks by President Trump, Vice President Pence, and members of the coronavirus task force in press briefing, March 15, 2020. Washington, DC: Office of the President of the United States; 2020. https://trumpwhitehouse.archives.gov/briefings-statements/remarks-presid...
-
- US Department of Health and Human Services. HHS continues Community Based Testing Sites for COVID-19 [Press release]. Washington, DC: US Department of Health and Human Services; 2021. https://www.hhs.gov/about/news/2021/01/07/hhs-continues-community-based-...
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

