MicroRNA-133b Inhibition Restores EGFR Expression and Accelerates Diabetes-Impaired Wound Healing
- PMID: 34873433
- PMCID: PMC8643265
- DOI: 10.1155/2021/9306760
MicroRNA-133b Inhibition Restores EGFR Expression and Accelerates Diabetes-Impaired Wound Healing
Retraction in
-
Retracted: MicroRNA-133b Inhibition Restores EGFR Expression and Accelerates Diabetes-Impaired Wound Healing.Oxid Med Cell Longev. 2024 Jan 9;2024:9893472. doi: 10.1155/2024/9893472. eCollection 2024. Oxid Med Cell Longev. 2024. PMID: 38234561 Free PMC article.
Abstract
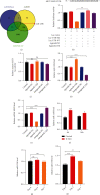
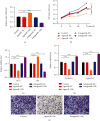
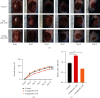
Diabetic foot ulcers (DFUs) are caused by impairments in peripheral blood vessel angiogenesis and represent a great clinical challenge. Although various innovative techniques and drugs have been developed for treating DFUs, therapeutic outcomes remain unsatisfactory. Using the GEO database, we obtained transcriptomic microarray data for DFUs and control wounds and detected a significant downregulation of epidermal growth factor receptor (EGFR) in DFUs. We cultured human umbilical vein endothelial cells (HUVECs) and noted downregulated EGFR expression following high-glucose exposure in vitro. Further, we observed decreased HUVEC proliferation and migration and increased apoptosis after shRNA-mediated EGFR silencing in these cells. In mice, EGFR inhibition via focal EGFR-shRNA injection delayed wound healing. Target prediction analysis followed by dual-luciferase reporter assays indicated that microRNA-133b (miR-133b) is a putative upstream regulator of EGFR expression. Increased miR-133b expression was observed in both glucose-treated HUVECs and wounds from diabetes patients, but no such change was observed in controls. miR-133b suppression enhanced the proliferation and angiogenic potential of cultured HUVECs and also accelerated wound healing. Although angiogenesis is not the sole mechanism affected in DFU, these findings suggest that the miR-133b-induced downregulation of EGFR may contribute to delayed wound healing in diabetes. Hence, miR-133b inhibition may be a useful strategy for treating diabetic wounds.
Copyright © 2021 Haobo Zhong et al.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures
Similar articles
-
Ginsenoside (Rg-1) promoted the wound closure of diabetic foot ulcer through iNOS elevation via miR-23a/IRF-1 axis.Life Sci. 2019 Sep 15;233:116525. doi: 10.1016/j.lfs.2019.05.081. Epub 2019 May 31. Life Sci. 2019. PMID: 31158376
-
Ginsenoside Rg1 promoted the wound healing in diabetic foot ulcers via miR-489-3p/Sirt1 axis.J Pharmacol Sci. 2021 Nov;147(3):271-283. doi: 10.1016/j.jphs.2021.07.008. Epub 2021 Aug 5. J Pharmacol Sci. 2021. PMID: 34507636
-
MiR-221-3p targets HIPK2 to promote diabetic wound healing.Microvasc Res. 2022 Mar;140:104306. doi: 10.1016/j.mvr.2021.104306. Epub 2021 Dec 30. Microvasc Res. 2022. PMID: 34973299
-
Role of microRNAs in diabetic foot ulcers: Mechanisms and possible interventions.Diabetes Res Clin Pract. 2024 Nov;217:111858. doi: 10.1016/j.diabres.2024.111858. Epub 2024 Sep 14. Diabetes Res Clin Pract. 2024. PMID: 39284457 Review.
-
Angio-microRNAs in diabetic foot ulcer-: Mechanistic insights and clinical perspectives.Prog Biophys Mol Biol. 2024 Oct;192:1-10. doi: 10.1016/j.pbiomolbio.2024.07.006. Epub 2024 Jul 27. Prog Biophys Mol Biol. 2024. PMID: 39069213 Review.
Cited by
-
Deciphering The Emerging Role of Programmed Cell Death in Diabetic Wound Healing.Int J Biol Sci. 2023 Sep 18;19(15):4989-5003. doi: 10.7150/ijbs.88461. eCollection 2023. Int J Biol Sci. 2023. PMID: 37781514 Free PMC article. Review.
-
Identifying miRNA Signatures Associated with Pancreatic Islet Dysfunction in a FOXA2-Deficient iPSC Model.Stem Cell Rev Rep. 2024 Oct;20(7):1915-1931. doi: 10.1007/s12015-024-10752-0. Epub 2024 Jun 25. Stem Cell Rev Rep. 2024. PMID: 38916841 Free PMC article.
-
Non-coding RNAs: Role in diabetic foot and wound healing.World J Diabetes. 2022 Dec 15;13(12):1001-1013. doi: 10.4239/wjd.v13.i12.1001. World J Diabetes. 2022. PMID: 36578864 Free PMC article. Review.
-
Innovative Treatment Strategies to Accelerate Wound Healing: Trajectory and Recent Advancements.Cells. 2022 Aug 6;11(15):2439. doi: 10.3390/cells11152439. Cells. 2022. PMID: 35954282 Free PMC article. Review.
-
Novel Insights into the Role of Keratinocytes-Expressed TRPV3 in the Skin.Biomolecules. 2023 Mar 10;13(3):513. doi: 10.3390/biom13030513. Biomolecules. 2023. PMID: 36979447 Free PMC article. Review.
References
-
- Nilforoushzadeh M. A., Kazemikhoo N., Mokmeli S., et al. An open-label study of low-level laser therapy followed by autologous fibroblast transplantation for healing grade 3 burn wounds in diabetic patients. Journal of Lasers in Medical Sciences . 2019;10(5):S7–S12. doi: 10.15171/jlms.2019.S2. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

