Ecology, evolution and spillover of coronaviruses from bats
- PMID: 34799704
- PMCID: PMC8603903
- DOI: 10.1038/s41579-021-00652-2
Ecology, evolution and spillover of coronaviruses from bats
Erratum in
-
Author Correction: Ecology, evolution and spillover of coronaviruses from bats.Nat Rev Microbiol. 2022 May;20(5):315. doi: 10.1038/s41579-022-00691-3. Nat Rev Microbiol. 2022. PMID: 35027705 Free PMC article. No abstract available.
Abstract
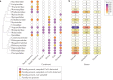
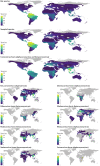
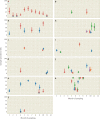
In the past two decades, three coronaviruses with ancestral origins in bats have emerged and caused widespread outbreaks in humans, including severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Since the first SARS epidemic in 2002-2003, the appreciation of bats as key hosts of zoonotic coronaviruses has advanced rapidly. More than 4,000 coronavirus sequences from 14 bat families have been identified, yet the true diversity of bat coronaviruses is probably much greater. Given that bats are the likely evolutionary source for several human coronaviruses, including strains that cause mild upper respiratory tract disease, their role in historic and future pandemics requires ongoing investigation. We review and integrate information on bat-coronavirus interactions at the molecular, tissue, host and population levels. We identify critical gaps in knowledge of bat coronaviruses, which relate to spillover and pandemic risk, including the pathways to zoonotic spillover, the infection dynamics within bat reservoir hosts, the role of prior adaptation in intermediate hosts for zoonotic transmission and the viral genotypes or traits that predict zoonotic capacity and pandemic potential. Filling these knowledge gaps may help prevent the next pandemic.
© 2021. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Epidemiology and Genomic Characterization of Two Novel SARS-Related Coronaviruses in Horseshoe Bats from Guangdong, China.mBio. 2022 Jun 28;13(3):e0046322. doi: 10.1128/mbio.00463-22. Epub 2022 Apr 25. mBio. 2022. PMID: 35467426 Free PMC article.
-
The Role of Molossidae and Vespertilionidae in Shaping the Diversity of Alphacoronaviruses in the Americas.Microbiol Spectr. 2022 Dec 21;10(6):e0314322. doi: 10.1128/spectrum.03143-22. Epub 2022 Oct 12. Microbiol Spectr. 2022. PMID: 36222689 Free PMC article.
-
[Source of the COVID-19 pandemic: ecology and genetics of coronaviruses (Betacoronavirus: Coronaviridae) SARS-CoV, SARS-CoV-2 (subgenus Sarbecovirus), and MERS-CoV (subgenus Merbecovirus).].Vopr Virusol. 2020;65(2):62-70. doi: 10.36233/0507-4088-2020-65-2-62-70. Vopr Virusol. 2020. PMID: 32515561 Review. Russian.
-
Spike-Independent Infection of Human Coronavirus 229E in Bat Cells.Microbiol Spectr. 2023 Jun 15;11(3):e0348322. doi: 10.1128/spectrum.03483-22. Epub 2023 May 18. Microbiol Spectr. 2023. PMID: 37199653 Free PMC article.
-
Coronaviruses in humans and animals: the role of bats in viral evolution.Environ Sci Pollut Res Int. 2021 Apr;28(16):19589-19600. doi: 10.1007/s11356-021-12553-1. Epub 2021 Mar 2. Environ Sci Pollut Res Int. 2021. PMID: 33655480 Free PMC article. Review.
Cited by
-
Oral Sampling of Little Brown Bat (Myotis lucifugus) Maternity Colonies for SARS-CoV-2 in the Northeast and Mid-Atlantic, USA.Animals (Basel). 2023 Feb 4;13(4):550. doi: 10.3390/ani13040550. Animals (Basel). 2023. PMID: 36830336 Free PMC article.
-
Monitoring SARS-CoV-2 Seroprevalence in Domestics and Exotic Animals in Southern France.Trop Med Infect Dis. 2023 Aug 25;8(9):426. doi: 10.3390/tropicalmed8090426. Trop Med Infect Dis. 2023. PMID: 37755888 Free PMC article.
-
Ecological countermeasures to prevent pathogen spillover and subsequent pandemics.Nat Commun. 2024 Mar 26;15(1):2577. doi: 10.1038/s41467-024-46151-9. Nat Commun. 2024. PMID: 38531842 Free PMC article. Review.
-
Co-circulation of alpha- and beta-coronaviruses in Pteropus vampyrus flying foxes from Indonesia.Transbound Emerg Dis. 2022 Nov;69(6):3917-3925. doi: 10.1111/tbed.14762. Epub 2022 Dec 2. Transbound Emerg Dis. 2022. PMID: 36382687 Free PMC article.
-
Location, Age, and Antibodies Predict Avian Influenza Virus Shedding in Ring-Billed and Franklin's Gulls in Minnesota.Animals (Basel). 2024 Sep 26;14(19):2781. doi: 10.3390/ani14192781. Animals (Basel). 2024. PMID: 39409730 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

