Connecting copper and cancer: from transition metal signalling to metalloplasia
- PMID: 34764459
- PMCID: PMC8810673
- DOI: 10.1038/s41568-021-00417-2
Connecting copper and cancer: from transition metal signalling to metalloplasia
Abstract
Copper is an essential nutrient whose redox properties make it both beneficial and toxic to the cell. Recent progress in studying transition metal signalling has forged new links between researchers of different disciplines that can help translate basic research in the chemistry and biology of copper into clinical therapies and diagnostics to exploit copper-dependent disease vulnerabilities. This concept is particularly relevant in cancer, as tumour growth and metastasis have a heightened requirement for this metal nutrient. Indeed, the traditional view of copper as solely an active site metabolic cofactor has been challenged by emerging evidence that copper is also a dynamic signalling metal and metalloallosteric regulator, such as for copper-dependent phosphodiesterase 3B (PDE3B) in lipolysis, mitogen-activated protein kinase kinase 1 (MEK1) and MEK2 in cell growth and proliferation and the kinases ULK1 and ULK2 in autophagy. In this Perspective, we summarize our current understanding of the connection between copper and cancer and explore how challenges in the field could be addressed by using the framework of cuproplasia, which is defined as regulated copper-dependent cell proliferation and is a representative example of a broad range of metalloplasias. Cuproplasia is linked to a diverse array of cellular processes, including mitochondrial respiration, antioxidant defence, redox signalling, kinase signalling, autophagy and protein quality control. Identifying and characterizing new modes of copper-dependent signalling offers translational opportunities that leverage disease vulnerabilities to this metal nutrient.
© 2021. Springer Nature Limited.
Conflict of interest statement
Competing interests
N.K.T. is a member of the Scientific Advisory Board of DepYmed Inc. V.M.G. is listed as an inventor on the patent application PCT/US2019/041571 submitted by Texas A&M University entitled “Compositions for the treatment of copper deficiency and methods of use”. D.C.B. holds ownership in Merlon Inc. A.I.B. holds equity in Alterity Biotechnology Ltd, Cogstate Ltd, Mesoblast Ltd and Collaborative Medicinal Development LLC and is a paid consultant for Collaborative Medicinal Development Pty Ltd. L.T.V. is a consultant for Berg Pharma, Osmol Therapeutics and Sema4, serves on the advisory board of Seattle Genetics and Immunomedics/Gilead, and receives research funding from Genentech, Arvinas, and Oncotheraphy Sciences. E.J.G, A.C., P.A.C., J.R.C, G.M.D., Q.P.D., K.J.F., S.G., S.G.K., S.L., V.M., M.J.P., R.P., M.R., M.L.S., L.V.A., D.X., P.Y. and C.J.C. declare no competing interests.
Figures
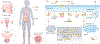
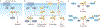

Similar articles
-
Copper is an essential regulator of the autophagic kinases ULK1/2 to drive lung adenocarcinoma.Nat Cell Biol. 2020 Apr;22(4):412-424. doi: 10.1038/s41556-020-0481-4. Epub 2020 Mar 16. Nat Cell Biol. 2020. PMID: 32203415 Free PMC article.
-
Targeting cuproplasia and cuproptosis in cancer.Nat Rev Clin Oncol. 2024 May;21(5):370-388. doi: 10.1038/s41571-024-00876-0. Epub 2024 Mar 14. Nat Rev Clin Oncol. 2024. PMID: 38486054 Review.
-
Cope with copper: From copper linked mechanisms to copper-based clinical cancer therapies.Cancer Lett. 2023 May 1;561:216157. doi: 10.1016/j.canlet.2023.216157. Epub 2023 Apr 1. Cancer Lett. 2023. PMID: 37011869 Review.
-
Metalloallostery and Transition Metal Signaling: Bioinorganic Copper Chemistry Beyond Active Sites.Angew Chem Int Ed Engl. 2023 Mar 6;62(11):e202213644. doi: 10.1002/anie.202213644. Epub 2023 Jan 18. Angew Chem Int Ed Engl. 2023. PMID: 36653724 Free PMC article. Review.
-
The copper chaperone CCS facilitates copper binding to MEK1/2 to promote kinase activation.J Biol Chem. 2021 Dec;297(6):101314. doi: 10.1016/j.jbc.2021.101314. Epub 2021 Oct 27. J Biol Chem. 2021. PMID: 34715128 Free PMC article.
Cited by
-
FDX1 as a novel biomarker and treatment target for stomach adenocarcinoma.World J Gastrointest Surg. 2024 Jun 27;16(6):1803-1824. doi: 10.4240/wjgs.v16.i6.1803. World J Gastrointest Surg. 2024. PMID: 38983344 Free PMC article.
-
Multi-omics analysis of the prognostic and biological role of cuproptosis-related gene in gastric cancer.J Gastrointest Oncol. 2024 Jun 30;15(3):946-962. doi: 10.21037/jgo-23-946. Epub 2024 Jun 19. J Gastrointest Oncol. 2024. PMID: 38989420 Free PMC article.
-
Dietary copper intake and the prevalence of kidney stones among adult in the United States: A propensity score matching study.Front Public Health. 2022 Aug 30;10:973887. doi: 10.3389/fpubh.2022.973887. eCollection 2022. Front Public Health. 2022. PMID: 36111192 Free PMC article.
-
Integrated bioinformatics and experiment revealed that cuproptosis is the potential common pathogenesis of three kinds of primary cardiomyopathy.Aging (Albany NY). 2023 Dec 11;15(23):14210-14241. doi: 10.18632/aging.205298. Epub 2023 Dec 11. Aging (Albany NY). 2023. PMID: 38085668 Free PMC article.
-
Comprehensive analysis of cuproptosis-related genes in prognosis, tumor microenvironment infiltration, and immunotherapy response in gastric cancer.J Cancer Res Clin Oncol. 2023 Jul;149(8):5453-5468. doi: 10.1007/s00432-022-04474-4. Epub 2022 Dec 3. J Cancer Res Clin Oncol. 2023. PMID: 36462036
References
-
- Hanahan D & Weinberg RA Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011). - PubMed
-
- Lippard SJ, Berg JM Principles of bioinorganic chemistry. vol. xvii, 411 p (University Science Books; 1994).
-
- Solomon EI, Sundaram UM & Machonkin TE Multicopper oxidases and oxygenases. Chem. Rev 96, 2563–2606 (1996). - PubMed
-
- Que EL, Domaille DW & Chang CJ Metals in neurobiology: probing their chemistry and biology with molecular imaging. Chem. Rev 108, 1517–1549 (2008). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA257254/CA/NCI NIH HHS/United States
- R01 DK116859/DK/NIDDK NIH HHS/United States
- R01 NS109307/NS/NINDS NIH HHS/United States
- P30 ES013508/ES/NIEHS NIH HHS/United States
- R01 DK071865/DK/NIDDK NIH HHS/United States
- R01 GM084176/GM/NIGMS NIH HHS/United States
- R01 CA053840/CA/NCI NIH HHS/United States
- R01 GM079465/GM/NIGMS NIH HHS/United States
- P30 CA013330/CA/NCI NIH HHS/United States
- R01 DK124907/DK/NIDDK NIH HHS/United States
- R01 GM101502/GM/NIGMS NIH HHS/United States
- R01 GM111672/GM/NIGMS NIH HHS/United States
- UL1 TR001863/TR/NCATS NIH HHS/United States
- P30 DK041296/DK/NIDDK NIH HHS/United States
- R21 GM129592/GM/NIGMS NIH HHS/United States
- R01 DK117396/DK/NIDDK NIH HHS/United States
- R01 CA190265/CA/NCI NIH HHS/United States
- U54 CA210184/CA/NCI NIH HHS/United States
- R35 GM124749/GM/NIGMS NIH HHS/United States
- R01 DK071111/DK/NIDDK NIH HHS/United States
- P30 CA008748/CA/NCI NIH HHS/United States
- P30 DK020541/DK/NIDDK NIH HHS/United States
- R01 GM120211/GM/NIGMS NIH HHS/United States
- R01 GM139245/GM/NIGMS NIH HHS/United States
- R21 CA184788/CA/NCI NIH HHS/United States
- P30 CA045508/CA/NCI NIH HHS/United States
- Z01 HD008768/ImNIH/Intramural NIH HHS/United States
- R37 CA053840/CA/NCI NIH HHS/United States

