DOK3 maintains intestinal homeostasis by suppressing JAK2/STAT3 signaling and S100a8/9 production in neutrophils
- PMID: 34743196
- PMCID: PMC8572282
- DOI: 10.1038/s41419-021-04357-5
DOK3 maintains intestinal homeostasis by suppressing JAK2/STAT3 signaling and S100a8/9 production in neutrophils
Abstract
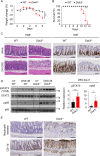
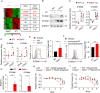
How pathogenesis of inflammatory bowel disease (IBD) depends on the complex interplay of host genetics, microbiome and the immune system is not fully understood. Here, we showed that Downstream of Kinase 3 (DOK3), an adapter protein involved in immune signaling, confers protection of mice from dextran sodium sulfate (DSS)-induced colitis. DOK3-deficiency promotes gut microbial dysbiosis and enhanced colitis susceptibility, which can be reversed by the transfer of normal microbiota from wild-type mice. Mechanistically, DOK3 exerts its protective effect by suppressing JAK2/STAT3 signaling in colonic neutrophils to limit their S100a8/9 production, thereby maintaining gut microbial ecology and colon homeostasis. Hence, our findings reveal that the immune system and microbiome function in a feed-forward manner, whereby DOK3 maintains colonic neutrophils in a quiescent state to establish a gut microbiome essential for intestinal homeostasis and protection from IBD.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Cmtm4 deficiency exacerbates colitis by inducing gut dysbiosis and S100a8/9 expression.J Genet Genomics. 2024 Aug;51(8):811-823. doi: 10.1016/j.jgg.2024.03.009. Epub 2024 Apr 2. J Genet Genomics. 2024. PMID: 38575111
-
STAT3 genotypic variation and cellular STAT3 activation and colon leukocyte recruitment in pediatric Crohn disease.J Pediatr Gastroenterol Nutr. 2012 Jul;55(1):32-43. doi: 10.1097/MPG.0b013e318246be78. J Pediatr Gastroenterol Nutr. 2012. PMID: 22197944 Free PMC article.
-
Interleukin-6 induces S100A9 expression in colonic epithelial cells through STAT3 activation in experimental ulcerative colitis.PLoS One. 2012;7(9):e38801. doi: 10.1371/journal.pone.0038801. Epub 2012 Sep 4. PLoS One. 2012. PMID: 22962574 Free PMC article.
-
S100A8 and S100A9 Are Important for Postnatal Development of Gut Microbiota and Immune System in Mice and Infants.Gastroenterology. 2020 Dec;159(6):2130-2145.e5. doi: 10.1053/j.gastro.2020.08.019. Epub 2020 Aug 15. Gastroenterology. 2020. PMID: 32805279
-
A Cross-Talk Between Microbiota-Derived Short-Chain Fatty Acids and the Host Mucosal Immune System Regulates Intestinal Homeostasis and Inflammatory Bowel Disease.Inflamm Bowel Dis. 2018 Feb 15;24(3):558-572. doi: 10.1093/ibd/izx029. Inflamm Bowel Dis. 2018. PMID: 29462379 Review.
Cited by
-
Expression and clinical significance of DOK3 in renal clear cell carcinoma.J Int Med Res. 2023 May;51(5):3000605231174974. doi: 10.1177/03000605231174974. J Int Med Res. 2023. PMID: 37235715 Free PMC article.
-
The emerging role of neutrophil extracellular traps in ulcerative colitis.Front Immunol. 2024 Aug 7;15:1425251. doi: 10.3389/fimmu.2024.1425251. eCollection 2024. Front Immunol. 2024. PMID: 39170617 Free PMC article. Review.
-
Dok3 restrains neutrophil production of calprotectin during TLR4 sensing of SARS-CoV-2 spike protein.Front Immunol. 2022 Sep 12;13:996637. doi: 10.3389/fimmu.2022.996637. eCollection 2022. Front Immunol. 2022. PMID: 36172386 Free PMC article.
-
Construction of a neural network diagnostic model and investigation of immune infiltration characteristics for Crohn's disease.Front Genet. 2022 Sep 15;13:976578. doi: 10.3389/fgene.2022.976578. eCollection 2022. Front Genet. 2022. PMID: 36186439 Free PMC article.
-
Fecal microbiota transplantation inhibits colorectal cancer progression: Reversing intestinal microbial dysbiosis to enhance anti-cancer immune responses.Front Microbiol. 2023 Apr 18;14:1126808. doi: 10.3389/fmicb.2023.1126808. eCollection 2023. Front Microbiol. 2023. PMID: 37143538 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

