Matrix-Degrading Enzyme Expression and Aortic Fibrosis During Continuous-Flow Left Ventricular Mechanical Support
- PMID: 34711337
- PMCID: PMC8562886
- DOI: 10.1016/j.jacc.2021.08.047
Matrix-Degrading Enzyme Expression and Aortic Fibrosis During Continuous-Flow Left Ventricular Mechanical Support
Abstract
Background: The effects of nonphysiological flow generated by continuous-flow (CF) left ventricular assist devices (LVADs) on the aorta remain poorly understood.
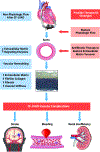
Objectives: The authors sought to quantify indexes of fibrosis and determine the molecular signature of post-CF-LVAD vascular remodeling.
Methods: Paired aortic tissue was collected at CF-LVAD implant and subsequently at transplant from 22 patients. Aortic wall morphometry and fibrillar collagen content (a measure of fibrosis) was quantified. In addition, whole-transcriptome profiling by RNA sequencing and follow-up immunohistochemistry were performed to evaluate CF-LVAD-mediated changes in aortic mRNA and protein expression.
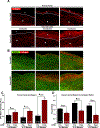
Results: The mean age was 52 ± 12 years, with a mean duration of CF-LVAD of 224 ± 193 days (range 45-798 days). There was a significant increase in the thickness of the collagen-rich adventitial layer from 218 ± 110 μm pre-LVAD to 410 ± 209 μm post-LVAD (P < 0.01). Furthermore, there was an increase in intimal and medial mean fibrillar collagen intensity from 22 ± 11 a.u. pre-LVAD to 41 ± 24 a.u. post-LVAD (P < 0.0001). The magnitude of this increase in fibrosis was greater among patients with longer durations of CF-LVAD support. CF-LVAD led to profound down-regulation in expression of extracellular matrix-degrading enzymes, such as matrix metalloproteinase-19 and ADAMTS4, whereas no evidence of fibroblast activation was noted.
Conclusions: There is aortic remodeling and fibrosis after CF-LVAD that correlates with the duration of support. This fibrosis is due, at least in part, to suppression of extracellular matrix-degrading enzyme expression. Further research is needed to examine the contribution of nonphysiological flow patterns on vascular function and whether modulation of pulsatility may improve vascular remodeling and long-term outcomes.
Keywords: aorta; congestive heart failure; fibrosis; left ventricular assist device; mechanical circulatory support; vascular remodeling.
Copyright © 2021 American College of Cardiology Foundation. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Funding Support and Author Disclosures REDCap was provided by National Institutes of Health/NCATS Colorado CTSA Grant Number UL1 TR002535. Imaging experiments were performed in the University of Colorado Anschutz Medical Campus Advanced Light Microscopy Core supported in part by National Institutes of Health/NCATS Colorado CTSI Grant Number UL1 TR002535. Dr Ambardekar was supported by a Scientist Development Grant from the American Heart Association and by the Boettcher Foundation’s Webb-Waring Biomedical Research Program. Dr Stratton was supported by National Institutes of Health grants HL126354 and AG056848. Dr Weiser-Evans was supported by the National Heart, Lung, and Blood Institute National Institutes of Health Grant Numbers R01 HL121877 and R01 HL123616. Dr McKinsey was supported by the National Institute of Health (grants HL116848, HL147558, DK119594, HL127240, and HL150225) and by the American Heart Association (16SFRN31400013); has received support from the Colorado Office of Economic Development and International Trade (CTGGI 19-3579) through the University of Colorado SPARK Program; is on the scientific advisory boards of Artemes Bio, Inc., and Eikonizo Therapeutics; has received funding from Italfarmaco for an unrelated project, and has a subcontract from Eikonizo Therapeutics related to an SBIR grant from the National Institutes of Health (HL154959). The contents are the authors’ sole responsibility and do not necessarily represent official National Institutes of Health views. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures




Comment in
-
Aortic Smooth Muscle Detraining in Continuous Flow LVAD: Out of Practice.J Am Coll Cardiol. 2021 Nov 2;78(18):1796-1799. doi: 10.1016/j.jacc.2021.08.045. J Am Coll Cardiol. 2021. PMID: 34711338 No abstract available.
-
Aortic Valve Remodeling in CF-LVAD: Beyond the Arterial Wall.J Am Coll Cardiol. 2022 Mar 29;79(12):e221-e222. doi: 10.1016/j.jacc.2022.01.026. J Am Coll Cardiol. 2022. PMID: 35331420 No abstract available.
-
Reply: Aortic Valve Remodeling in CF-LVAD: Beyond the Arterial Wall.J Am Coll Cardiol. 2022 Mar 29;79(12):e223. doi: 10.1016/j.jacc.2022.01.027. J Am Coll Cardiol. 2022. PMID: 35331421 No abstract available.
Similar articles
-
Coronary Artery Remodeling and Fibrosis With Continuous-Flow Left Ventricular Assist Device Support.Circ Heart Fail. 2018 May;11(5):e004491. doi: 10.1161/CIRCHEARTFAILURE.117.004491. Circ Heart Fail. 2018. PMID: 29724722 Free PMC article.
-
Morphologic changes in the aortic wall media after support with a continuous-flow left ventricular assist device.J Heart Lung Transplant. 2013 Nov;32(11):1096-100. doi: 10.1016/j.healun.2013.07.007. Epub 2013 Aug 19. J Heart Lung Transplant. 2013. PMID: 23968812
-
Dynamic Changes in Aortic Vascular Stiffness in Patients Bridged to Transplant With Continuous-Flow Left Ventricular Assist Devices.JACC Heart Fail. 2017 Jun;5(6):449-459. doi: 10.1016/j.jchf.2016.12.009. Epub 2017 Mar 8. JACC Heart Fail. 2017. PMID: 28285118
-
Continuous-Flow Left Ventricular Assist Devices and Valvular Heart Disease: A Comprehensive Review.Can J Cardiol. 2020 Feb;36(2):244-260. doi: 10.1016/j.cjca.2019.11.022. Epub 2019 Nov 25. Can J Cardiol. 2020. PMID: 32036866 Review.
-
Hypertension: an unstudied potential risk factor for adverse outcomes during continuous flow ventricular assist device support.Heart Fail Rev. 2015 May;20(3):317-22. doi: 10.1007/s10741-014-9458-3. Heart Fail Rev. 2015. PMID: 25283767 Free PMC article. Review.
Cited by
-
Pulsatility and flow patterns across macro- and microcirculatory arteries of continuous-flow left ventricular assist device patients.J Heart Lung Transplant. 2023 Sep;42(9):1223-1232. doi: 10.1016/j.healun.2023.04.002. Epub 2023 Apr 23. J Heart Lung Transplant. 2023. PMID: 37098374 Free PMC article.
-
CH-VAD left ventricular assist implantation combined with the Bentall procedure and coronary artery bypass grafting.ESC Heart Fail. 2024 Aug;11(4):2405-2409. doi: 10.1002/ehf2.14769. Epub 2024 Mar 23. ESC Heart Fail. 2024. PMID: 38520316 Free PMC article.
-
Analysis of the Genetic Relationship between Atherosclerosis and Non-Alcoholic Fatty Liver Disease through Biological Interaction Networks.Int J Mol Sci. 2023 Feb 18;24(4):4124. doi: 10.3390/ijms24044124. Int J Mol Sci. 2023. PMID: 36835545 Free PMC article.
-
Cellular and Molecular Mechanisms Activated by a Left Ventricular Assist Device.Int J Mol Sci. 2023 Dec 24;25(1):288. doi: 10.3390/ijms25010288. Int J Mol Sci. 2023. PMID: 38203459 Free PMC article. Review.
-
Transcriptomics, metabolomics, and in-silico drug predictions for liver damage in young and aged burn victims.Commun Biol. 2023 Jun 2;6(1):597. doi: 10.1038/s42003-023-04964-2. Commun Biol. 2023. PMID: 37268765 Free PMC article.
References
-
- Cornwell WK 3rd, Tarumi T, Stickford A et al. Restoration of Pulsatile Flow Reduces Sympathetic Nerve Activity Among Individuals With Continuous-Flow Left Ventricular Assist Devices. Circulation 2015;132:2316–22. - PubMed
-
- Mandelbaum I, Burns WH. Pulsatile and Nonpulsatile Blood Flow. JAMA 1965;191:657–60. - PubMed
-
- Patel AC, Dodson RB, Cornwell WK 3rd et al. Dynamic Changes in Aortic Vascular Stiffness in Patients Bridged to Transplant With Continuous-Flow Left Ventricular Assist Devices. JACC Heart Fail 2017;5:449–459. - PubMed
-
- Ambardekar AV, Hunter KS, Babu AN, Tuder RM, Dodson RB, Lindenfeld J. Changes in Aortic Wall Structure, Composition, and Stiffness With Continuous-Flow Left Ventricular Assist Devices: A Pilot Study. Circ Heart Fail 2015;8:944–52. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K01 AG056848/AG/NIA NIH HHS/United States
- R43 HL154959/HL/NHLBI NIH HHS/United States
- 16SFRN31400013/AHA/American Heart Association-American Stroke Association/United States
- R01 HL116848/HL/NHLBI NIH HHS/United States
- R01 HL127240/HL/NHLBI NIH HHS/United States
- R01 HL147558/HL/NHLBI NIH HHS/United States
- T32 GM007635/GM/NIGMS NIH HHS/United States
- R01 HL150225/HL/NHLBI NIH HHS/United States
- R01 DK119594/DK/NIDDK NIH HHS/United States
- UL1 TR002535/TR/NCATS NIH HHS/United States
- R01 HL121877/HL/NHLBI NIH HHS/United States
- R01 HL123616/HL/NHLBI NIH HHS/United States
- T32 GM008497/GM/NIGMS NIH HHS/United States
- F32 HL126354/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

