Cirrhosis regression is associated with improved clinical outcomes in patients with nonalcoholic steatohepatitis
- PMID: 34662449
- PMCID: PMC9303958
- DOI: 10.1002/hep.32204
Cirrhosis regression is associated with improved clinical outcomes in patients with nonalcoholic steatohepatitis
Abstract
Background and aims: Surrogate endpoints that predict complications are necessary for assessment and approval of NASH therapies. We assessed associations between histologic and noninvasive tests (NITs) of fibrosis with liver-related complications in patients with NASH cirrhosis.
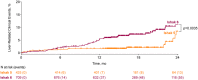
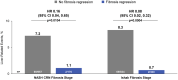
Approach and results: Patients with compensated cirrhosis due to NASH were enrolled in two placebo-controlled trials of simtuzumab and selonsertib. Liver fibrosis at baseline and week 48 (W48) was staged by NASH Clinical Research Network (CRN) and Ishak classifications and a machine learning (ML) approach, hepatic collagen and alpha-smooth muscle actin (α-SMA) expression were quantified by morphometry, liver stiffness (LS) was measured by transient elastography, and serum NITs (enhanced liver fibrosis [ELF], NAFLD fibrosis score [NFS], and Fibrosis-4 index [FIB-4]) were calculated. Cox regression determined associations between these parameters at baseline and their changes over time with adjudicated liver-related clinical events. Among 1,135 patients, 709 (62%) had Ishak stage 6 fibrosis, and median ELF and LS were 10.66 and 21.1 kPa, respectively. During a median follow-up of 16.6 months, 71 (6.3%) had a liver-related event; associated baseline factors included Ishak stage 6 fibrosis, and higher hepatic collagen, α-SMA expression, ML-based fibrosis parameters, LS, ELF, NFS, and FIB-4. Cirrhosis regression observed in 16% (176/1,135) between BL and W48 was associated with a lower risk of events versus nonregression (1.1% [2/176] vs. 7.2% [69/957]; HR, 0.16; 95% CI, 0.04, 0.65 [p = 0.0104]). Conversely, after adjustment for baseline values, increases in hepatic collagen, α-SMA, ML-based fibrosis parameters, NFS, and LS were associated with an increased risk of events.
Conclusions: In patients with compensated cirrhosis due to NASH, regression of fibrosis is associated with a reduction in liver-related complications. These data support the utility of histologic fibrosis regression and NITs as clinical trial endpoints for NASH cirrhosis.
© 2022 The Authors. Hepatology published by Wiley Periodicals LLC on behalf of American Association for the Study of Liver Diseases.
Conflict of interest statement
Dr. Myers is employed by and owns stock in Gilead. Dr. Afdhal advises Gilead and Echosens. Dr. Anstee consults for, is on the speakers’ bureau for, has active research collaborations with, and received grants from Allergan/Tobira. He consults for, has active research collaborations with, and received grants from AstraZeneca, Novartis, and Pfizer. He consults for, is on the speakers’ bureau for, and has active research collaborations with Bristol‐Myers Squibb and Genfit. He consults for and is on the speakers’ bureau for Abbott and Gilead. He consults for and has active research collaborations with Eli Lilly, HistoIndex, Intercept, and Novo Nordisk. He has active research collaborations with and received grants from AbbVie, GlaxoSmithKline, and Glympse Bio. He consults for 89Bio, Acuitas, Altimmune, Axcella, Blade, BNN Cardio, Celgene, Cirius, CymaBay, EcoR1, E3Bio, Galmed, Genentech, Grunthal, Indalo, Imperial Innovations, Inventiva, IQVIA, Janssen, Madrigal, Medimmune, Metacrine, NewGene, NGMBio, North Sea, Poxel, ProSciento, Raptor, Servier, Terns, and Viking. He has active research collaborations with Antaros, Boehringer Ingelheim, Echosens, Ellegaard Gottingen Minipigs AS, Exalenz, iXscient, Nordic Bioscience, One Way Liver Genomics, Perspectum, Resoundant, Sanofi‐Aventis, SomaLogic, and Takeda. He is on the speakers’ bureau for Clinical Care Options, Falk, Fishawack, Integritas, Kenes, and MedScape. He received grants from Vertex. He received royalties from Elsevier. Dr. Sanyal consults and received grants from Conatus, Gilead, Mallinckrodt, Immuron, Boehringer Ingelheim, Novartis, Bristol‐Myers Squibb, Merck, Eli Lilly, Novo Nordisk, Fractyl, Siemens, Madrigal, Inventiva, and Covance. He consults for and owns stock in Genfit. He consults for Intercept, Pfizer, Salix, Galectin, Hemoshear, Terns, Albireo, Sanofi, Janssen, Takeda, NorthSea, AMRA, Perspectum, Poxel, 89 Bio, AstraZeneca, NGM, Amgen, Regeneron, Genentech, Roche, Prosciento, Histoindex, Path AI, and Biocellvia. He received grants from Echosens‐Sandhill, Owl, and Second Genome. He owns stock in Exalenz, Sanyal Bio, Durect, Indalo, Tiziana, and Rivus. He received royalties from Elsevier and UptoDate. Dr. Trauner advises for, is on the speakers’ bureau for, and received grants from Gilead, Intercept, and MSD. He advises and received grants from Falk and Albireo. He advises BiomX, Boehringer Ingelheim, Genfit, Janssen, Novartis, Phenex, Regulus, and Shire. He is on the speakers’ bureau for Falk Foundation. He received grants from AbbVie, Alnylam, Cymabay, Takeda, and UltraGenyx. Dr. Lawitz received grants from 89Bio, Akero, Alnylam, Amgen, AstraZeneca, Axcella, Boehringer Ingelheim, Bristol‐Myers Squibb, Cymabay, CytoDyn, Durect, Eli Lilly, Enanta, Galectin, Galmed, Genentech, Gilead, Hanmi, Intercept, Inventiva, Janssen, Laboratory for Advanced Medicine, Madrigal, Merck, Metacrine, NGM, NorthSea, Novartis, Novo Nordisk, Pfizer, Poxel, Roche, Sagimet, Synlogic, Terns, Valeant, Viking, and Zydus. Dr. Abdelmalek received grants from Gilead. Dr. Ding is employed by and owns stock in Gilead. Dr. Jia is employed by and owns stock in Gilead. Dr. Huss is employed by and owns stock in Gilead. Dr. Chung is employed by and owns stock in Gilead. Dr. Wong consults for and received grants from Gilead. He consults for 3V‐Bio, AbbVie, Allergan, Boehringer Ingelheim, Echosens, Inventiva, Merck, Novartis, Novo Nordisk, Pfizer, ProSciento, Sagimet, TARGET, and Terns. He is cofounder of Illuminatio Medical Technology Limited. Dr. Muir consults for and received grants from Gilead. He received grants from Cymabay. Dr. Bosch consults for Gilead and Actelion. Dr. Younossi consults for Gilead, Intercept, Novo Nordisk, Siemens, and Terns. Dr. Harrison consults for, advises, received grants from, and owns stock in Akero, Galectin, Genfit, Hepion, Metacrine, NGM, and NorthSea. He consults for, advises, and received grants from Axcella, Civi, CymaBay, Gilead, High Tide, Intercept, Madrigal, Novartis, Novo Nordisk, Poxel, and Sagimet. He consults for, advises, and owns stock in HistoIndex and Sonic Incytes. He consults for and advises Altimmune, Echosens, Foresite, Medpace, Prometic, Ridgeline, and Terns. He consults for and received grants from Enyo and Viking. He advises and received grants from Galmed. He advises and owns stock in Chronwell and PathAI. He received grants from and owns stock in Cirius. He consults for AgomAB, Alentis, Alimentiv, Boston Pharmaceuticals, B Riley, BVF, Canfite, Corcept, Fibronostics, Fortress, Inipharm, Ionis, Kowa, Microba, Nutrasource, and Piper Sandler. He advises 89Bio, Arrowhead, Indalo, and Theratechnologies.
Figures

Comment in
-
Letter to the editor: In patients with established NASH cirrhosis, is there no role of weight reduction in reversing fibrosis?Hepatology. 2022 Jul;76(1):E3-E4. doi: 10.1002/hep.32375. Epub 2022 Feb 15. Hepatology. 2022. PMID: 35092319 No abstract available.
-
Letter to the editor: Spontaneous regression of cirrhosis: A paradigm shift in our understanding of the natural history of NASH.Hepatology. 2022 Jul;76(1):E1-E2. doi: 10.1002/hep.32374. Epub 2022 Mar 4. Hepatology. 2022. PMID: 35102586 No abstract available.
Similar articles
-
Sequential Diagnostic Approach Using FIB-4 and ELF for Predicting Advanced Fibrosis in Metabolic Dysfunction-Associated Steatotic Liver Disease.Diagnostics (Basel). 2024 Nov 11;14(22):2517. doi: 10.3390/diagnostics14222517. Diagnostics (Basel). 2024. PMID: 39594183 Free PMC article.
-
Nonalcoholic Fatty Liver Disease and Staging of Hepatic Fibrosis.Adv Exp Med Biol. 2024;1460:539-574. doi: 10.1007/978-3-031-63657-8_18. Adv Exp Med Biol. 2024. PMID: 39287864 Review.
-
Falls prevention interventions for community-dwelling older adults: systematic review and meta-analysis of benefits, harms, and patient values and preferences.Syst Rev. 2024 Nov 26;13(1):289. doi: 10.1186/s13643-024-02681-3. Syst Rev. 2024. PMID: 39593159 Free PMC article.
-
Infliximab for maintenance of medically-induced remission in Crohn's disease.Cochrane Database Syst Rev. 2024 Feb 19;2(2):CD012609. doi: 10.1002/14651858.CD012609.pub2. Cochrane Database Syst Rev. 2024. PMID: 38372447 Free PMC article. Review.
-
Antioxidants for female subfertility.Cochrane Database Syst Rev. 2020 Aug 27;8(8):CD007807. doi: 10.1002/14651858.CD007807.pub4. Cochrane Database Syst Rev. 2020. PMID: 32851663 Free PMC article.
Cited by
-
Surveillance of the progression and assessment of treatment endpoints for nonalcoholic steatohepatitis.Clin Mol Hepatol. 2023 Feb;29(Suppl):S228-S243. doi: 10.3350/cmh.2022.0401. Epub 2022 Dec 14. Clin Mol Hepatol. 2023. PMID: 36521452 Free PMC article. Review.
-
Increases and decreases in liver stiffness measurement are independently associated with the risk of liver-related events in NAFLD.J Hepatol. 2024 Oct;81(4):600-608. doi: 10.1016/j.jhep.2024.05.008. Epub 2024 May 16. J Hepatol. 2024. PMID: 38762169
-
KASL clinical practice guidelines for noninvasive tests to assess liver fibrosis in chronic liver disease.Clin Mol Hepatol. 2024 Sep;30(Suppl):S5-S105. doi: 10.3350/cmh.2024.0506. Epub 2024 Aug 19. Clin Mol Hepatol. 2024. PMID: 39159947 Free PMC article. No abstract available.
-
Challenges and opportunities in NASH drug development.Nat Med. 2023 Mar;29(3):562-573. doi: 10.1038/s41591-023-02242-6. Epub 2023 Mar 9. Nat Med. 2023. PMID: 36894650 Review.
-
Recompensation in cirrhosis: unravelling the evolving natural history of nonalcoholic fatty liver disease.Nat Rev Gastroenterol Hepatol. 2024 Jan;21(1):46-56. doi: 10.1038/s41575-023-00846-4. Epub 2023 Oct 5. Nat Rev Gastroenterol Hepatol. 2024. PMID: 37798441 Review.
References
-
- Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet. 2014;383(9930):1749–61. - PubMed
-
- Taylor RS, Taylor RJ, Bayliss S, Hagström H, Nasr P, Schattenberg JM, et al. Association between fibrosis stage and outcomes of patients with nonalcoholic fatty liver disease: a systematic review and meta‐analysis. Gastroenterology. 2020;158(6):1611–25.e12. - PubMed
-
- Sanyal AJ, Harrison SA, Ratziu V, Abdelmalek MF, Diehl AM, Caldwell S, et al. The natural history of advanced fibrosis due to nonalcoholic steatohepatitis: data from the simtuzumab trials. Hepatology. 2019;70(6):1913–27. - PubMed
-
- Vilar‐Gomez E, Calzadilla‐Bertot L, Wai‐Sun Wong V, Castellanos M, Aller‐de la Fuente R, Metwally M, et al. Fibrosis severity as a determinant of cause‐specific mortality in patients with advanced nonalcoholic fatty liver disease: a multi‐national cohort study. Gastroenterology. 2018;155(2):443–57.e17. - PubMed
-
- Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313–21. - PubMed

