A Phase II Study to Evaluate the Safety and Efficacy of Prasinezumab in Early Parkinson's Disease (PASADENA): Rationale, Design, and Baseline Data
- PMID: 34659081
- PMCID: PMC8518716
- DOI: 10.3389/fneur.2021.705407
A Phase II Study to Evaluate the Safety and Efficacy of Prasinezumab in Early Parkinson's Disease (PASADENA): Rationale, Design, and Baseline Data
Abstract
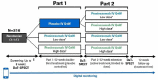
Background: Currently available treatments for Parkinson's disease (PD) do not slow clinical progression nor target alpha-synuclein, a key protein associated with the disease. Objective: The study objective was to evaluate the efficacy and safety of prasinezumab, a humanized monoclonal antibody that binds aggregated alpha-synuclein, in individuals with early PD. Methods: The PASADENA study is a multicenter, randomized, double-blind, placebo-controlled treatment study. Individuals with early PD, recruited across the US and Europe, received monthly intravenous doses of prasinezumab (1,500 or 4,500 mg) or placebo for a 52-week period (Part 1), followed by a 52-week extension (Part 2) in which all participants received active treatment. Key inclusion criteria were: aged 40-80 years; Hoehn & Yahr (H&Y) Stage I or II; time from diagnosis ≤2 years; having bradykinesia plus one other cardinal sign of PD (e.g., resting tremor, rigidity); DAT-SPECT imaging consistent with PD; and either treatment naïve or on a stable monoamine oxidase B (MAO-B) inhibitor dose. Study design assumptions for sample size and study duration were built using a patient cohort from the Parkinson's Progression Marker Initiative (PPMI). In this report, baseline characteristics are compared between the treatment-naïve and MAO-B inhibitor-treated PASADENA cohorts and between the PASADENA and PPMI populations. Results: Of the 443 patients screened, 316 were enrolled into the PASADENA study between June 2017 and November 2018, with an average age of 59.9 years and 67.4% being male. Mean time from diagnosis at baseline was 10.11 months, with 75.3% in H&Y Stage II. Baseline motor and non-motor symptoms (assessed using Movement Disorder Society-Unified Parkinson's Disease Rating Scale [MDS-UPDRS]) were similar in severity between the MAO-B inhibitor-treated and treatment-naïve PASADENA cohorts (MDS-UPDRS sum of Parts I + II + III [standard deviation (SD)]; 30.21 [11.96], 32.10 [13.20], respectively). The overall PASADENA population (63.6% treatment naïve and 36.4% on MAO-B inhibitor) showed a similar severity in MDS-UPDRS scores (e.g., MDS-UPDRS sum of Parts I + II + III [SD]; 31.41 [12.78], 32.63 [13.04], respectively) to the PPMI cohort (all treatment naïve). Conclusions: The PASADENA study population is suitable to investigate the potential of prasinezumab to slow disease progression in individuals with early PD. Trial Registration: NCT03100149.
Keywords: MDS-UPDRS = Movement Disorder Society—Unified Parkinson's Disease Rating Scale; Parkinson's disease; Phase II clinical trial; alpha-synuclein (α-syn); disease modification treatments; disease progression; monoclonal antibodies; prasinezumab.
Copyright © 2021 Pagano, Boess, Taylor, Ricci, Mollenhauer, Poewe, Boulay, Anzures-Cabrera, Vogt, Marchesi, Post, Nikolcheva, Kinney, Zago, Ness, Svoboda, Britschgi, Ostrowitzki, Simuni, Marek, Koller, Sevigny, Doody, Fontoura, Umbricht, Bonni and PASADENA Investigators and Prasinezumab Study Group.
Conflict of interest statement
GP, FB, KT, BR, JA-C, AV, MM, TN, HS, MB, SO, RD, DU, and ABon are employees of F. Hoffmann-La Roche Ltd. ABou is an employee of Idorsia Pharmaceuticals Ltd. AP is an employee of Feetme SAS. JS is an employee of Prevail Therapeutics. GK, WZ, DN, and MK are employees of Prothena Biosciences Inc. The authors declare that F. Hoffmann-La Roche Ltd was the sponsor of the study. F. Hoffmann-La Roche Ltd and Prothena Corporation plc were the funders of the study. F. Hoffmann-La Roche Ltd and Prothena Corporation plc were involved in the study design, collection, analysis, interpretation of data, the writing of this article and the decision to submit it for publication. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Sustained effect of prasinezumab on Parkinson's disease motor progression in the open-label extension of the PASADENA trial.Nat Med. 2024 Dec;30(12):3669-3675. doi: 10.1038/s41591-024-03270-6. Epub 2024 Oct 8. Nat Med. 2024. PMID: 39379705 Free PMC article. Clinical Trial.
-
A Phase 2b, multicenter, randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of intravenous prasinezumab in early-stage Parkinson's disease (PADOVA): Rationale, design, and baseline data.Parkinsonism Relat Disord. 2024 Dec 29;132:107257. doi: 10.1016/j.parkreldis.2024.107257. Online ahead of print. Parkinsonism Relat Disord. 2024. PMID: 39798255
-
Prasinezumab slows motor progression in rapidly progressing early-stage Parkinson's disease.Nat Med. 2024 Apr;30(4):1096-1103. doi: 10.1038/s41591-024-02886-y. Epub 2024 Apr 15. Nat Med. 2024. PMID: 38622249 Free PMC article.
-
Does levodopa slow or hasten the rate of progression of Parkinson's disease?J Neurol. 2005 Oct;252 Suppl 4:IV37-IV42. doi: 10.1007/s00415-005-4008-5. J Neurol. 2005. PMID: 16222436 Review.
-
Comparative efficacy and safety of monoamine oxidase type B inhibitors plus channel blockers and monoamine oxidase type B inhibitors as adjuvant therapy to levodopa in the treatment of Parkinson's disease: a network meta-analysis of randomized controlled trials.Eur J Neurol. 2023 Apr;30(4):1118-1134. doi: 10.1111/ene.15651. Epub 2022 Dec 11. Eur J Neurol. 2023. PMID: 36437702 Review.
Cited by
-
Rational Generation of Monoclonal Antibodies Selective for Pathogenic Forms of Alpha-Synuclein.Biomedicines. 2022 Sep 2;10(9):2168. doi: 10.3390/biomedicines10092168. Biomedicines. 2022. PMID: 36140270 Free PMC article.
-
Parkinson's disease therapy: what lies ahead?J Neural Transm (Vienna). 2023 Jun;130(6):793-820. doi: 10.1007/s00702-023-02641-6. Epub 2023 May 5. J Neural Transm (Vienna). 2023. PMID: 37147404 Free PMC article. Review.
-
Sustained effect of prasinezumab on Parkinson's disease motor progression in the open-label extension of the PASADENA trial.Nat Med. 2024 Dec;30(12):3669-3675. doi: 10.1038/s41591-024-03270-6. Epub 2024 Oct 8. Nat Med. 2024. PMID: 39379705 Free PMC article. Clinical Trial.
-
Diagnosis Versus Classification of Essential Tremor: A Research Perspective.J Mov Disord. 2023 May;16(2):152-157. doi: 10.14802/jmd.23020. Epub 2023 May 24. J Mov Disord. 2023. PMID: 37258278 Free PMC article. No abstract available.
-
Modeling of Parkinson's Disease Progression and Implications for Detection of Disease Modification in Treatment Trials.J Parkinsons Dis. 2024;14(6):1225-1235. doi: 10.3233/JPD-230446. J Parkinsons Dis. 2024. PMID: 39058452 Free PMC article.
References
-
- Bonnet AM. Involvement of non-dopaminergic pathways in Parkinson's disease. CNS Drugs. (2000) 13:351–64. 10.2165/00023210-200013050-00005 - DOI
Associated data
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

