The SARS CoV-2 spike directed non-neutralizing polyclonal antibodies cross-react with Human immunodeficiency virus (HIV-1) gp41
- PMID: 34649114
- PMCID: PMC8479463
- DOI: 10.1016/j.intimp.2021.108187
The SARS CoV-2 spike directed non-neutralizing polyclonal antibodies cross-react with Human immunodeficiency virus (HIV-1) gp41
Abstract
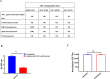
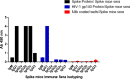
Cross-reactivity among the two diverse viruses is believed to originate from the concept of antibodies recognizing similar epitopes on the two viral surfaces. Cross-reactive antibody responses have been seen in previous variants of SARS and SARS-CoV-2, but little is known about the cross reactivity with other similar RNA viruses like HIV-1. In the present study, we examined the reactivity the SARS-CoV-2 directed antibodies, via spike, immunized mice sera and demonstrated whether they conferred any cross-reactive neutralization against HIV-1. Our findings show that SARS-CoV-2 spike immunized mice antibodies cross-react with the HIV-1 Env protein. Cross-neutralization among the two viruses is uncommon, suggesting the presence of a non-neutralizing antibody response to conserved epitopes amongst the two viruses. Our results indicate, that SARS-CoV-2 spike antibody cross reactivity is targeted towards the gp41 region of the HIV-1 Env (gp160) protein. Overall, our investigation not only answers a crucial question about the understanding of cross-reactive epitopes of antibodies generated in different viral infections, but also provides critical evidence for developing vaccine immunogens and novel treatment strategies with enhanced efficacy capable of recognising diverse pathogens with similar antigenic features.
Keywords: Class I viruses; Cross-reactivity; Gp41; HIV-1; Non-neutralizing antibodies; SARS-CoV2.
Copyright © 2021 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





Similar articles
-
Non-neutralizing SARS CoV-2 directed polyclonal antibodies demonstrate cross-reactivity with the HA glycans of influenza virus.Int Immunopharmacol. 2021 Oct;99:108020. doi: 10.1016/j.intimp.2021.108020. Epub 2021 Jul 29. Int Immunopharmacol. 2021. PMID: 34426117 Free PMC article.
-
Escape from neutralizing antibodies by SARS-CoV-2 spike protein variants.Elife. 2020 Oct 28;9:e61312. doi: 10.7554/eLife.61312. Elife. 2020. PMID: 33112236 Free PMC article.
-
Lack of antibody-mediated cross-protection between SARS-CoV-2 and SARS-CoV infections.EBioMedicine. 2020 Aug;58:102890. doi: 10.1016/j.ebiom.2020.102890. Epub 2020 Jul 21. EBioMedicine. 2020. PMID: 32707445 Free PMC article.
-
Cross-reactive human immunodeficiency virus type 1-neutralizing human monoclonal antibody that recognizes a novel conformational epitope on gp41 and lacks reactivity against self-antigens.J Virol. 2008 Jul;82(14):6869-79. doi: 10.1128/JVI.00033-08. Epub 2008 May 14. J Virol. 2008. PMID: 18480433 Free PMC article.
-
Cross-reactive Antibody Response between SARS-CoV-2 and SARS-CoV Infections.Cell Rep. 2020 Jun 2;31(9):107725. doi: 10.1016/j.celrep.2020.107725. Epub 2020 May 18. Cell Rep. 2020. PMID: 32426212 Free PMC article.
Cited by
-
Self-DNA driven inflammation in COVID-19 and after mRNA-based vaccination: lessons for non-COVID-19 pathologies.Front Immunol. 2024 Feb 19;14:1259879. doi: 10.3389/fimmu.2023.1259879. eCollection 2023. Front Immunol. 2024. PMID: 38439942 Free PMC article.
-
Triple Burden: The Incorrigible Threat of Tuberculosis, HIV, and COVID-19.Infect Disord Drug Targets. 2024;24(4):1-7. doi: 10.2174/0118715265259959231031104820. Infect Disord Drug Targets. 2024. PMID: 37937570 Review.
-
HIV and SARS-CoV-2 Co-Infection: From Population Study Evidence to In Vitro Studies.Life (Basel). 2022 Dec 13;12(12):2089. doi: 10.3390/life12122089. Life (Basel). 2022. PMID: 36556453 Free PMC article. Review.
-
Non-neutralizing antibodies: Deleterious or propitious during SARS-CoV-2 infection?Int Immunopharmacol. 2022 Sep;110:108943. doi: 10.1016/j.intimp.2022.108943. Epub 2022 Jun 13. Int Immunopharmacol. 2022. PMID: 35753123 Free PMC article. Review.
-
Characterization and immunogenicity assessment of MERS-CoV pre-fusion spike trimeric oligomers as vaccine immunogen.Hum Vaccin Immunother. 2024 Dec 31;20(1):2351664. doi: 10.1080/21645515.2024.2351664. Epub 2024 May 17. Hum Vaccin Immunother. 2024. PMID: 38757508 Free PMC article.
References
-
- Structures and Mechanisms of Viral Membrane Fusion Proteins, n.d.. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2649671/ (accessed March 17, 2021).
-
- Gallaher: Model of the pre-insertion region of the... - Google विद्वान, (n.d.). https://scholar.google.com/scholar_lookup?journal=Virology&title=Model+o... (accessed May 12, 2021).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

