Nuclear-localized human respiratory syncytial virus NS1 protein modulates host gene transcription
- PMID: 34644581
- PMCID: PMC8609347
- DOI: 10.1016/j.celrep.2021.109803
Nuclear-localized human respiratory syncytial virus NS1 protein modulates host gene transcription
Abstract
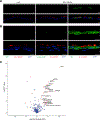
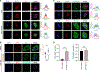
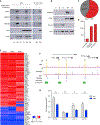
Human respiratory syncytial virus (RSV) is a common cause of lower respiratory tract infections in the pediatric, elderly, and immunocompromised individuals. RSV non-structural protein NS1 is a known cytosolic immune antagonist, but how NS1 modulates host responses remains poorly defined. Here, we observe NS1 partitioning into the nucleus of RSV-infected cells, including the human airway epithelium. Nuclear NS1 coimmunoprecipitates with Mediator complex and is chromatin associated. Chromatin-immunoprecipitation demonstrates enrichment of NS1 that overlaps Mediator and transcription factor binding within the promoters and enhancers of differentially expressed genes during RSV infection. Mutation of the NS1 C-terminal helix reduces NS1 impact on host gene expression. These data suggest that nuclear NS1 alters host responses to RSV infection by binding at regulatory elements of immune response genes and modulating host gene transcription. Our study identifies another layer of regulation by virally encoded proteins that shapes host response and impacts immunity to RSV.
Keywords: NS1; epigenetics; host gene transcription; immune antagonism; non-structural protein; respiratory syncytial virus.
Copyright © 2021 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures

Similar articles
-
An Unexpected Encounter: Respiratory Syncytial Virus Nonstructural Protein 1 Interacts with Mediator Subunit MED25.J Virol. 2022 Oct 12;96(19):e0129722. doi: 10.1128/jvi.01297-22. Epub 2022 Sep 14. J Virol. 2022. PMID: 36102648 Free PMC article. Review.
-
Human respiratory syncytial virus non-structural protein NS1 modifies miR-24 expression via transforming growth factor-β.J Gen Virol. 2015 Nov;96(11):3179-3191. doi: 10.1099/jgv.0.000261. J Gen Virol. 2015. PMID: 26253191 Free PMC article.
-
The interactome of the human respiratory syncytial virus NS1 protein highlights multiple effects on host cell biology.J Virol. 2012 Aug;86(15):7777-89. doi: 10.1128/JVI.00460-12. Epub 2012 May 16. J Virol. 2012. PMID: 22593156 Free PMC article.
-
Effects of altering the transcription termination signals of respiratory syncytial virus on viral gene expression and growth in vitro and in vivo.J Virol. 2004 Jan;78(2):692-9. doi: 10.1128/jvi.78.2.692-699.2004. J Virol. 2004. PMID: 14694100 Free PMC article.
-
Respiratory syncytial virus nonstructural proteins 1 and 2: Exceptional disrupters of innate immune responses.PLoS Pathog. 2019 Oct 17;15(10):e1007984. doi: 10.1371/journal.ppat.1007984. eCollection 2019 Oct. PLoS Pathog. 2019. PMID: 31622448 Free PMC article. Review.
Cited by
-
Respiratory Syncytial Virus (RSV) optimizes the translational landscape during infection.bioRxiv [Preprint]. 2024 Aug 3:2024.08.02.606199. doi: 10.1101/2024.08.02.606199. bioRxiv. 2024. PMID: 39131278 Free PMC article. Preprint.
-
The Role of the CX3CR1-CX3CL1 Axis in Respiratory Syncytial Virus Infection and the Triggered Immune Response.Int J Mol Sci. 2024 Sep 11;25(18):9800. doi: 10.3390/ijms25189800. Int J Mol Sci. 2024. PMID: 39337288 Free PMC article. Review.
-
Host Responses to Respiratory Syncytial Virus Infection.Viruses. 2023 Sep 26;15(10):1999. doi: 10.3390/v15101999. Viruses. 2023. PMID: 37896776 Free PMC article. Review.
-
Antagonism between viral infection and innate immunity at the single-cell level.PLoS Pathog. 2023 Sep 5;19(9):e1011597. doi: 10.1371/journal.ppat.1011597. eCollection 2023 Sep. PLoS Pathog. 2023. PMID: 37669278 Free PMC article.
-
Comparative analysis of retroviral Gag-host cell interactions: focus on the nuclear interactome.Retrovirology. 2024 Jun 19;21(1):13. doi: 10.1186/s12977-024-00645-y. Retrovirology. 2024. PMID: 38898526 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

