Engineering of fully humanized and vascularized 3D bone marrow niches sustaining undifferentiated human cord blood hematopoietic stem and progenitor cells
- PMID: 34616539
- PMCID: PMC8488506
- DOI: 10.1177/20417314211044855
Engineering of fully humanized and vascularized 3D bone marrow niches sustaining undifferentiated human cord blood hematopoietic stem and progenitor cells
Abstract
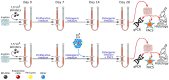
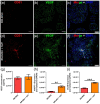
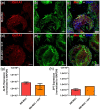
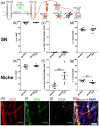
Hematopoietic stem and progenitor cells (HSPCs) are frequently located around the bone marrow (BM) vasculature. These so-called perivascular niches regulate HSC function both in health and disease, but they have been poorly studied in humans due to the scarcity of models integrating complete human vascular structures. Herein, we propose the stromal vascular fraction (SVF) derived from human adipose tissue as a cell source to vascularize 3D osteoblastic BM niches engineered in perfusion bioreactors. We show that SVF cells form self-assembled capillary structures, composed by endothelial and perivascular cells, that add to the osteogenic matrix secreted by BM mesenchymal stromal cells in these engineered niches. In comparison to avascular osteoblastic niches, vascularized BM niches better maintain immunophenotypically-defined cord blood (CB) HSCs without affecting cell proliferation. In contrast, HSPCs cultured in vascularized BM niches showed increased CFU-granulocyte-erythrocyte-monocyte-megakaryocyte (CFU-GEMM) numbers. The vascularization also contributed to better preserve osteogenic gene expression in the niche, demonstrating that niche vascularization has an influence on both hematopoietic and stromal compartments. In summary, we have engineered a fully humanized and vascularized 3D BM tissue to model native human endosteal perivascular niches and revealed functional implications of this vascularization in sustaining undifferentiated CB HSPCs. This system provides a unique modular platform to explore hemato-vascular interactions in human healthy/pathological hematopoiesis.
Keywords: Hematopoietic stem cell; bone marrow microenvironment; engineered 3D niches; human hematopoiesis; perivascular niche.
© The Author(s) 2021.
Conflict of interest statement
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
Similar articles
-
Culturing patient-derived malignant hematopoietic stem cells in engineered and fully humanized 3D niches.Proc Natl Acad Sci U S A. 2021 Oct 5;118(40):e2114227118. doi: 10.1073/pnas.2114227118. Proc Natl Acad Sci U S A. 2021. PMID: 34580200 Free PMC article.
-
Bioengineering the human bone marrow microenvironment in liquefied compartments: A promising approach for the recapitulation of osteovascular niches.Acta Biomater. 2022 Sep 1;149:167-178. doi: 10.1016/j.actbio.2022.07.001. Epub 2022 Jul 8. Acta Biomater. 2022. PMID: 35811072
-
In vitro biomimetic engineering of a human hematopoietic niche with functional properties.Proc Natl Acad Sci U S A. 2018 Jun 19;115(25):E5688-E5695. doi: 10.1073/pnas.1805440115. Epub 2018 Jun 4. Proc Natl Acad Sci U S A. 2018. PMID: 29866839 Free PMC article.
-
Current approaches in biomaterial-based hematopoietic stem cell niches.Acta Biomater. 2018 May;72:1-15. doi: 10.1016/j.actbio.2018.03.028. Epub 2018 Mar 22. Acta Biomater. 2018. PMID: 29578087 Review.
-
Hierarchy of immature hematopoietic cells related to blood flow and niche.Curr Opin Hematol. 2011 Jul;18(4):220-5. doi: 10.1097/MOH.0b013e3283475fe7. Curr Opin Hematol. 2011. PMID: 21519242 Review.
Cited by
-
Relationship between indices of circulating blood cells and bone homeostasis in osteoporosis.Front Endocrinol (Lausanne). 2022 Sep 5;13:965290. doi: 10.3389/fendo.2022.965290. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36133307 Free PMC article.
-
Bioengineered Living Bone Grafts-A Concise Review on Bioreactors and Production Techniques In Vitro.Int J Mol Sci. 2022 Feb 3;23(3):1765. doi: 10.3390/ijms23031765. Int J Mol Sci. 2022. PMID: 35163687 Free PMC article. Review.
-
Bone Marrow Niches of Hematopoietic Stem and Progenitor Cells.Int J Mol Sci. 2022 Apr 18;23(8):4462. doi: 10.3390/ijms23084462. Int J Mol Sci. 2022. PMID: 35457280 Free PMC article. Review.
-
A millifluidic bioreactor allows the long term culture of primary lymphocytes or CD34+ hematopoietic cells while allowing the detection of tumorigenic expansion.Front Bioeng Biotechnol. 2024 Oct 2;12:1388312. doi: 10.3389/fbioe.2024.1388312. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 39416278 Free PMC article.
-
Bone Marrow Niches and Tumour Cells: Lights and Shadows of a Mutual Relationship.Front Immunol. 2022 May 6;13:884024. doi: 10.3389/fimmu.2022.884024. eCollection 2022. Front Immunol. 2022. PMID: 35603212 Free PMC article. Review.
References
-
- Ramasamy SK, Kusumbe AP, Itkin T, et al.. Regulation of hematopoiesis and osteogenesis by blood vessel-derived signals. Annu Rev Cell Dev Biol 2016; 32: 649–675. - PubMed
LinkOut - more resources
Full Text Sources

