Waning of BNT162b2 Vaccine Protection against SARS-CoV-2 Infection in Qatar
- PMID: 34614327
- PMCID: PMC8522799
- DOI: 10.1056/NEJMoa2114114
Waning of BNT162b2 Vaccine Protection against SARS-CoV-2 Infection in Qatar
Abstract
Background: Waning of vaccine protection against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection or coronavirus disease 2019 (Covid-19) is a concern. The persistence of BNT162b2 (Pfizer-BioNTech) vaccine effectiveness against infection and disease in Qatar, where the B.1.351 (or beta) and B.1.617.2 (or delta) variants have dominated incidence and polymerase-chain-reaction testing is done on a mass scale, is unclear.
Methods: We used a matched test-negative, case-control study design to estimate vaccine effectiveness against any SARS-CoV-2 infection and against any severe, critical, or fatal case of Covid-19, from January 1 to September 5, 2021.
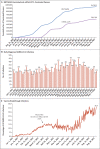
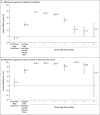
Results: Estimated BNT162b2 effectiveness against any SARS-CoV-2 infection was negligible in the first 2 weeks after the first dose. It increased to 36.8% (95% confidence interval [CI], 33.2 to 40.2) in the third week after the first dose and reached its peak at 77.5% (95% CI, 76.4 to 78.6) in the first month after the second dose. Effectiveness declined gradually thereafter, with the decline accelerating after the fourth month to reach approximately 20% in months 5 through 7 after the second dose. Effectiveness against symptomatic infection was higher than effectiveness against asymptomatic infection but waned similarly. Variant-specific effectiveness waned in the same pattern. Effectiveness against any severe, critical, or fatal case of Covid-19 increased rapidly to 66.1% (95% CI, 56.8 to 73.5) by the third week after the first dose and reached 96% or higher in the first 2 months after the second dose; effectiveness persisted at approximately this level for 6 months.
Conclusions: BNT162b2-induced protection against SARS-CoV-2 infection appeared to wane rapidly following its peak after the second dose, but protection against hospitalization and death persisted at a robust level for 6 months after the second dose. (Funded by Weill Cornell Medicine-Qatar and others.).
Copyright © 2021 Massachusetts Medical Society.
Figures
Similar articles
-
Effect of mRNA Vaccine Boosters against SARS-CoV-2 Omicron Infection in Qatar.N Engl J Med. 2022 May 12;386(19):1804-1816. doi: 10.1056/NEJMoa2200797. Epub 2022 Mar 9. N Engl J Med. 2022. PMID: 35263534 Free PMC article.
-
Effectiveness of two and three doses of COVID-19 mRNA vaccines against infection, symptoms, and severity in the pre-omicron era: A time-dependent gradient.Vaccine. 2024 May 22;42(14):3307-3320. doi: 10.1016/j.vaccine.2024.04.026. Epub 2024 Apr 14. Vaccine. 2024. PMID: 38616439
-
Effectiveness of mRNA vaccines and waning of protection against SARS-CoV-2 infection and severe covid-19 during predominant circulation of the delta variant in Italy: retrospective cohort study.BMJ. 2022 Feb 10;376:e069052. doi: 10.1136/bmj-2021-069052. BMJ. 2022. PMID: 35144968 Free PMC article.
-
BNT162b2 and mRNA-1273 COVID-19 vaccine effectiveness against the SARS-CoV-2 Delta variant in Qatar.Nat Med. 2021 Dec;27(12):2136-2143. doi: 10.1038/s41591-021-01583-4. Epub 2021 Nov 2. Nat Med. 2021. PMID: 34728831
-
Covid-19 Vaccine Protection among Children and Adolescents in Qatar.N Engl J Med. 2022 Nov 17;387(20):1865-1876. doi: 10.1056/NEJMoa2210058. Epub 2022 Nov 2. N Engl J Med. 2022. PMID: 36322837 Free PMC article.
Cited by
-
Antibody titers among healthcare workers for coronavirus disease 2019 at 6 months after BNT162b2 vaccination.Vaccine. 2022 Sep 16;40(39):5670-5674. doi: 10.1016/j.vaccine.2022.08.035. Epub 2022 Aug 22. Vaccine. 2022. PMID: 36030124 Free PMC article.
-
SARS-CoV-2-The Role of Natural Immunity: A Narrative Review.J Clin Med. 2022 Oct 25;11(21):6272. doi: 10.3390/jcm11216272. J Clin Med. 2022. PMID: 36362500 Free PMC article. Review.
-
Mathematical Optimization Strategy for Effectiveness Profile Estimation in Two-Dose Vaccines and Its Use in Designing Improved Vaccination Strategies Focused on Pandemic Containment.Vaccines (Basel). 2024 Jan 12;12(1):81. doi: 10.3390/vaccines12010081. Vaccines (Basel). 2024. PMID: 38250894 Free PMC article.
-
Simulating potential outbreaks of Delta and Omicron variants based on contact-tracing data: A modelling study in Fujian Province, China.Infect Dis Model. 2023 Mar;8(1):270-281. doi: 10.1016/j.idm.2023.02.002. Epub 2023 Feb 18. Infect Dis Model. 2023. PMID: 36846047 Free PMC article.
-
Factors Related to Non-compliance With Non-pharmaceutical Interventions to Mitigate the Spread of SARS-CoV-2: Results From a Survey in the Swiss General Adult Population.Front Public Health. 2022 Mar 25;10:828584. doi: 10.3389/fpubh.2022.828584. eCollection 2022. Front Public Health. 2022. PMID: 35400068 Free PMC article.
References
-
- Chemaitelly H, Yassine HM, Benslimane FM, et al. mRNA-1273 COVID-19 vaccine effectiveness against the B.1.1.7 and B.1.351 variants and severe COVID-19 disease in Qatar. Nat Med 2021;27:1614-1621. - PubMed
-
- Qatar Ministry of Public Health. National Covid-19 vaccination program data, 2021. (https://covid19.moph.gov.qa/EN/Pages/Vaccination-Program-Data.aspx).
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
