Biosynthetic Glycan Labeling
- PMID: 34606245
- PMCID: PMC8943913
- DOI: 10.1021/jacs.1c07430
Biosynthetic Glycan Labeling
Abstract
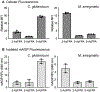
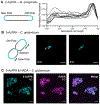
Glycans are ubiquitous and play important biological roles, yet chemical methods for probing their structure and function within cells remain limited. Strategies for studying other biomacromolecules, such as proteins, often exploit chemoselective reactions for covalent modification, capture, or imaging. Unlike amino acids that constitute proteins, glycan building blocks lack distinguishing reactivity because they are composed primarily of polyol isomers. Moreover, encoding glycan variants through genetic manipulation is complex. Therefore, we formulated a new, generalizable strategy for chemoselective glycan modification that directly takes advantage of cellular glycosyltransferases. Many of these enzymes are selective for the products they generate yet promiscuous in their donor preferences. Thus, we designed reagents with bioorthogonal handles that function as glycosyltransferase substrate surrogates. We validated the feasibility of this approach by synthesizing and testing probes of d-arabinofuranose (d-Araf), a monosaccharide found in bacteria and an essential component of the cell wall that protects mycobacteria, including Mycobacterium tuberculosis. The result is the first probe capable of selectively labeling arabinofuranose-containing glycans. Our studies serve as a platform for developing new chemoselective labeling agents for other privileged monosaccharides. This probe revealed an asymmetric distribution of d-Araf residues during mycobacterial cell growth and could be used to detect mycobacteria in THP1-derived macrophages.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

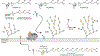

Similar articles
-
Biosynthetic incorporation for visualizing bacterial glycans.Methods Enzymol. 2022;665:135-151. doi: 10.1016/bs.mie.2021.12.005. Epub 2022 Jan 28. Methods Enzymol. 2022. PMID: 35379432
-
Bump-and-Hole Engineering Identifies Specific Substrates of Glycosyltransferases in Living Cells.Mol Cell. 2020 Jun 4;78(5):824-834.e15. doi: 10.1016/j.molcel.2020.03.030. Epub 2020 Apr 22. Mol Cell. 2020. PMID: 32325029 Free PMC article.
-
Direct imaging of glycans in Arabidopsis roots via click labeling of metabolically incorporated azido-monosaccharides.BMC Plant Biol. 2016 Oct 10;16(1):220. doi: 10.1186/s12870-016-0907-0. BMC Plant Biol. 2016. PMID: 27724898 Free PMC article.
-
How glycosylation affects glycosylation: the role of N-glycans in glycosyltransferase activity.Glycobiology. 2020 Dec 9;30(12):941-969. doi: 10.1093/glycob/cwaa041. Glycobiology. 2020. PMID: 32363402 Review.
-
Advances in cell surface glycoengineering reveal biological function.Glycobiology. 2016 Aug;26(8):789-96. doi: 10.1093/glycob/cww045. Epub 2016 Apr 10. Glycobiology. 2016. PMID: 27066802 Free PMC article. Review.
Cited by
-
Chemical tools to study bacterial glycans: a tale from discovery of glycoproteins to disruption of their function.Isr J Chem. 2023 Feb;63(1-2):e202200050. doi: 10.1002/ijch.202200050. Epub 2022 Oct 18. Isr J Chem. 2023. PMID: 37324574 Free PMC article.
-
ZnI2-Mediated cis-Glycosylations of Various Constrained Glycosyl Donors: Recent Advances in cis-Selective Glycosylations.Molecules. 2024 Oct 4;29(19):4710. doi: 10.3390/molecules29194710. Molecules. 2024. PMID: 39407638 Free PMC article. Review.
-
Selective Exoenzymatic Labeling of Lipooligosaccharides of Neisseria gonorrhoeae with α2,6-Sialoside Analogues.Chembiochem. 2022 Oct 6;23(19):e202200340. doi: 10.1002/cbic.202200340. Epub 2022 Aug 23. Chembiochem. 2022. PMID: 35877976 Free PMC article.
-
Profile of Carolyn R. Bertozzi, Morten Meldal, and K. Barry Sharpless: 2022 Nobel laureates in Chemistry.Proc Natl Acad Sci U S A. 2023 Aug 29;120(35):e2308367120. doi: 10.1073/pnas.2308367120. Epub 2023 Aug 21. Proc Natl Acad Sci U S A. 2023. PMID: 37603763 Free PMC article. No abstract available.
-
Azido Inositol Probes Enable Metabolic Labeling of Inositol-Containing Glycans and Reveal an Inositol Importer in Mycobacteria.ACS Chem Biol. 2023 Mar 17;18(3):595-604. doi: 10.1021/acschembio.2c00912. Epub 2023 Mar 1. ACS Chem Biol. 2023. PMID: 36856664 Free PMC article.
References
-
- Boutureira O; Bernardes GJ, Advances in chemical protein modification. Chem Rev 2015, 115, 2174–95. - PubMed
-
- Gunnoo SB; Madder A, Bioconjugation - using selective chemistry to enhance the properties of proteins and peptides as therapeutics and carriers. Org Biomol Chem 2016, 14, 8002–8013. - PubMed
-
- Cioce A; Bineva-Todd G; Agbay AJ; Choi J; Wood TM; Debets MF; Browne WM; Douglas HL; Roustan C; Tastan O; Kjaer S; Bush JT; Bertozzi CR; Schumann B, Metabolic Engineering Optimizes Bioorthogonal Glycan Labeling in Living Cells. 2021. ChemRxiv. DOI:10.26434/chemrxiv.13514365.v1. (accessed 2021-09-07) - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

