Structure-based classification of tauopathies
- PMID: 34588692
- PMCID: PMC7611841
- DOI: 10.1038/s41586-021-03911-7
Structure-based classification of tauopathies
Abstract
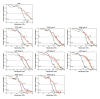
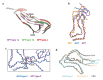
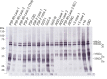
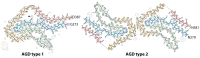
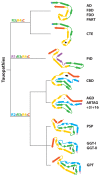
The ordered assembly of tau protein into filaments characterizes several neurodegenerative diseases, which are called tauopathies. It was previously reported that, by cryo-electron microscopy, the structures of tau filaments from Alzheimer's disease1,2, Pick's disease3, chronic traumatic encephalopathy4 and corticobasal degeneration5 are distinct. Here we show that the structures of tau filaments from progressive supranuclear palsy (PSP) define a new three-layered fold. Moreover, the structures of tau filaments from globular glial tauopathy are similar to those from PSP. The tau filament fold of argyrophilic grain disease (AGD) differs, instead resembling the four-layered fold of corticobasal degeneration. The AGD fold is also observed in ageing-related tau astrogliopathy. Tau protofilament structures from inherited cases of mutations at positions +3 or +16 in intron 10 of MAPT (the microtubule-associated protein tau gene) are also identical to those from AGD, suggesting that relative overproduction of four-repeat tau can give rise to the AGD fold. Finally, the structures of tau filaments from cases of familial British dementia and familial Danish dementia are the same as those from cases of Alzheimer's disease and primary age-related tauopathy. These findings suggest a hierarchical classification of tauopathies on the basis of their filament folds, which complements clinical diagnosis and neuropathology and also allows the identification of new entities-as we show for a case diagnosed as PSP, but with filament structures that are intermediate between those of globular glial tauopathy and PSP.
© 2021. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Figures






Comment in
-
Structural strains of misfolded tau protein define different diseases.Nature. 2021 Oct;598(7880):264-265. doi: 10.1038/d41586-021-02611-6. Nature. 2021. PMID: 34588645 No abstract available.
Similar articles
-
Novel tau filament fold in corticobasal degeneration.Nature. 2020 Apr;580(7802):283-287. doi: 10.1038/s41586-020-2043-0. Epub 2020 Feb 12. Nature. 2020. PMID: 32050258 Free PMC article.
-
Structures of filaments from Pick's disease reveal a novel tau protein fold.Nature. 2018 Sep;561(7721):137-140. doi: 10.1038/s41586-018-0454-y. Epub 2018 Aug 29. Nature. 2018. PMID: 30158706 Free PMC article.
-
Argyrophilic grain disease and Alzheimer's disease are distinguished by their different distribution of tau protein isoforms.Acta Neuropathol. 2002 Oct;104(4):425-34. doi: 10.1007/s00401-002-0591-z. Epub 2002 Jul 26. Acta Neuropathol. 2002. PMID: 12200631
-
Invited review: Neuropathology of tauopathies: principles and practice.Neuropathol Appl Neurobiol. 2015 Feb;41(1):3-23. doi: 10.1111/nan.12208. Neuropathol Appl Neurobiol. 2015. PMID: 25495175 Review.
-
Ordered Assembly of Tau Protein and Neurodegeneration.Adv Exp Med Biol. 2019;1184:3-21. doi: 10.1007/978-981-32-9358-8_1. Adv Exp Med Biol. 2019. PMID: 32096024 Review.
Cited by
-
Blood based biomarkers for movement disorders.Acta Neurol Scand. 2022 Oct;146(4):353-361. doi: 10.1111/ane.13700. Acta Neurol Scand. 2022. PMID: 36156206 Free PMC article. Review.
-
Massive experimental quantification of amyloid nucleation allows interpretable deep learning of protein aggregation.bioRxiv [Preprint]. 2024 Oct 1:2024.07.13.603366. doi: 10.1101/2024.07.13.603366. bioRxiv. 2024. PMID: 39071305 Free PMC article. Preprint.
-
The cellular prion protein does not affect tau seeding and spreading of sarkosyl-insoluble fractions from Alzheimer's disease.Sci Rep. 2024 Sep 16;14(1):21622. doi: 10.1038/s41598-024-72232-2. Sci Rep. 2024. PMID: 39284839 Free PMC article.
-
TAF15 amyloid filaments in frontotemporal lobar degeneration.Nature. 2024 Jan;625(7994):345-351. doi: 10.1038/s41586-023-06801-2. Epub 2023 Dec 6. Nature. 2024. PMID: 38057661 Free PMC article.
-
Tau reduction with artificial microRNAs modulates neuronal physiology and improves tauopathy phenotypes in mice.Mol Ther. 2024 Apr 3;32(4):1080-1095. doi: 10.1016/j.ymthe.2024.01.033. Epub 2024 Feb 3. Mol Ther. 2024. PMID: 38310353
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

