Efficacy of the mRNA-1273 SARS-CoV-2 Vaccine at Completion of Blinded Phase
- PMID: 34551225
- PMCID: PMC8482810
- DOI: 10.1056/NEJMoa2113017
Efficacy of the mRNA-1273 SARS-CoV-2 Vaccine at Completion of Blinded Phase
Abstract
Background: At interim analysis in a phase 3, observer-blinded, placebo-controlled clinical trial, the mRNA-1273 vaccine showed 94.1% efficacy in preventing coronavirus disease 2019 (Covid-19). After emergency use of the vaccine was authorized, the protocol was amended to include an open-label phase. Final analyses of efficacy and safety data from the blinded phase of the trial are reported.
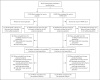
Methods: We enrolled volunteers who were at high risk for Covid-19 or its complications; participants were randomly assigned in a 1:1 ratio to receive two intramuscular injections of mRNA-1273 (100 μg) or placebo, 28 days apart, at 99 centers across the United States. The primary end point was prevention of Covid-19 illness with onset at least 14 days after the second injection in participants who had not previously been infected with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The data cutoff date was March 26, 2021.
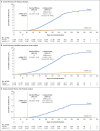
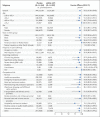
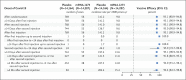
Results: The trial enrolled 30,415 participants; 15,209 were assigned to receive the mRNA-1273 vaccine, and 15,206 to receive placebo. More than 96% of participants received both injections, 2.3% had evidence of SARS-CoV-2 infection at baseline, and the median follow-up was 5.3 months in the blinded phase. Vaccine efficacy in preventing Covid-19 illness was 93.2% (95% confidence interval [CI], 91.0 to 94.8), with 55 confirmed cases in the mRNA-1273 group (9.6 per 1000 person-years; 95% CI, 7.2 to 12.5) and 744 in the placebo group (136.6 per 1000 person-years; 95% CI, 127.0 to 146.8). The efficacy in preventing severe disease was 98.2% (95% CI, 92.8 to 99.6), with 2 cases in the mRNA-1273 group and 106 in the placebo group, and the efficacy in preventing asymptomatic infection starting 14 days after the second injection was 63.0% (95% CI, 56.6 to 68.5), with 214 cases in the mRNA-1273 group and 498 in the placebo group. Vaccine efficacy was consistent across ethnic and racial groups, age groups, and participants with coexisting conditions. No safety concerns were identified.
Conclusions: The mRNA-1273 vaccine continued to be efficacious in preventing Covid-19 illness and severe disease at more than 5 months, with an acceptable safety profile, and protection against asymptomatic infection was observed. (Funded by the Biomedical Advanced Research and Development Authority and the National Institute of Allergy and Infectious Diseases; COVE ClinicalTrials.gov number, NCT04470427.).
Copyright © 2021 Massachusetts Medical Society.
Figures

Similar articles
-
Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine.N Engl J Med. 2021 Feb 4;384(5):403-416. doi: 10.1056/NEJMoa2035389. Epub 2020 Dec 30. N Engl J Med. 2021. PMID: 33378609 Free PMC article. Clinical Trial.
-
Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine through 6 Months.N Engl J Med. 2021 Nov 4;385(19):1761-1773. doi: 10.1056/NEJMoa2110345. Epub 2021 Sep 15. N Engl J Med. 2021. PMID: 34525277 Free PMC article. Clinical Trial.
-
Final efficacy analysis, interim safety analysis, and immunogenicity of a single dose of recombinant novel coronavirus vaccine (adenovirus type 5 vector) in adults 18 years and older: an international, multicentre, randomised, double-blinded, placebo-controlled phase 3 trial.Lancet. 2022 Jan 15;399(10321):237-248. doi: 10.1016/S0140-6736(21)02753-7. Epub 2021 Dec 23. Lancet. 2022. PMID: 34953526 Free PMC article. Clinical Trial.
-
Evaluation of mRNA-1273 Covid-19 Vaccine in Children 6 to 11 Years of Age.N Engl J Med. 2022 May 26;386(21):2011-2023. doi: 10.1056/NEJMoa2203315. Epub 2022 May 11. N Engl J Med. 2022. PMID: 35544369 Free PMC article. Clinical Trial.
-
Efficacy and safety of COVID-19 vaccines.Cochrane Database Syst Rev. 2022 Dec 7;12(12):CD015477. doi: 10.1002/14651858.CD015477. Cochrane Database Syst Rev. 2022. PMID: 36473651 Free PMC article. Review.
Cited by
-
Mucosal delivery of nanovaccine strategy against COVID-19 and its variants.Acta Pharm Sin B. 2022 Nov 21;13(7):2897-925. doi: 10.1016/j.apsb.2022.11.022. Online ahead of print. Acta Pharm Sin B. 2022. PMID: 36438851 Free PMC article. Review.
-
Risk for Facial Palsy after COVID-19 Vaccination, South Korea, 2021-2022.Emerg Infect Dis. 2024 Nov;30(11):2313-2322. doi: 10.3201/eid3011.240610. Epub 2024 Oct 8. Emerg Infect Dis. 2024. PMID: 39378869 Free PMC article.
-
A quick scoping review of the first year of vaccination against the COVID-19 pandemic: Do we need more shots or time?Medicine (Baltimore). 2022 Sep 16;101(37):e30609. doi: 10.1097/MD.0000000000030609. Medicine (Baltimore). 2022. PMID: 36123868 Free PMC article.
-
Efficacy, immunogenicity and safety of COVID-19 vaccines in older adults: a systematic review and meta-analysis.Front Immunol. 2022 Sep 13;13:965971. doi: 10.3389/fimmu.2022.965971. eCollection 2022. Front Immunol. 2022. PMID: 36177017 Free PMC article.
-
Associations of Immunogenicity and Reactogenicity After Severe Acute Respiratory Syndrome Coronavirus 2 mRNA-1273 Vaccine in the COVE and TeenCOVE Trials.Clin Infect Dis. 2023 Jan 13;76(2):271-280. doi: 10.1093/cid/ciac780. Clin Infect Dis. 2023. PMID: 36130187 Free PMC article.
References
-
- Johnson & Johnson COVID-19 vaccine authorized by U.S. FDA for emergency use — first single-shot vaccine in fight against global pandemic. New Brunswick, NJ: Johnson & Johnson, February 27, 2021. (https://www.jnj.com/johnson-johnson-covid-19-vaccine-authorized-by-u-s-f...).
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- UM1 AI148689/AI/NIAID NIH HHS/United States
- I01 BX003714/BX/BLRD VA/United States
- UM1 AI069424/AI/NIAID NIH HHS/United States
- UM1 AI148685/AI/NIAID NIH HHS/United States
- UM1 AI068618/AI/NIAID NIH HHS/United States
- UM1 AI148373/AI/NIAID NIH HHS/United States
- UM1 AI148684/AI/NIAID NIH HHS/United States
- L40 AI154610/AI/NIAID NIH HHS/United States
- UM1 AI068635/AI/NIAID NIH HHS/United States
- UM1 AI068619/AI/NIAID NIH HHS/United States
- K23 AI159399/AI/NIAID NIH HHS/United States
- UM1 AI068636/AI/NIAID NIH HHS/United States
- K08 AI143923/AI/NIAID NIH HHS/United States
- P30 ES010126/ES/NIEHS NIH HHS/United States
- UM1 AI148575/AI/NIAID NIH HHS/United States
- UM1 AI148576/AI/NIAID NIH HHS/United States
- UM1 AI068614/AI/NIAID NIH HHS/United States
- K12 HD052892/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
