A systematic review on global RSV genetic data: Identification of knowledge gaps
- PMID: 34543489
- PMCID: PMC9285027
- DOI: 10.1002/rmv.2284
A systematic review on global RSV genetic data: Identification of knowledge gaps
Abstract
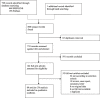
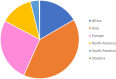
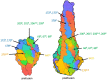
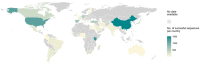
Respiratory syncytial virus (RSV) is a major health problem. A better understanding of the geographical and temporal dynamics of RSV circulation will assist in tracking resistance against therapeutics currently under development. Since 2015, the field of RSV molecular epidemiology has evolved rapidly with around 20-30 published articles per year. The objective of this systematic review is to identify knowledge gaps in recent RSV genetic literature to guide global molecular epidemiology research. We included 78 studies published between 2015 and 2020 describing 12,998 RSV sequences of which 8,233 (63%) have been uploaded to GenBank. Seventeen (22%) studies were performed in low- and middle-income countries (LMICs), and seven (9%) studies sequenced whole-genomes. Although most reported polymorphisms for monoclonal antibodies in clinical development (nirsevimab, MK-1654) have not been tested for resistance in neutralisation essays, known resistance was detected at low levels for the nirsevimab and palivizumab binding site. High resistance was found for the suptavumab binding site. We present the first literature review of an enormous amount of RSV genetic data. The need for global monitoring of RSV molecular epidemiology becomes increasingly important in evaluating the effectiveness of monoclonal antibody candidates as they reach their final stages of clinical development. We have identified the following three knowledge gaps: whole-genome data to study global RSV evolution, data from LMICs and data from global surveillance programs.
Keywords: RSV; molecular epidemiology; monoclonal antibodies; respiratory syncytial virus; systematic review.
© 2021 The Authors. Reviews in Medical Virology published by John Wiley & Sons Ltd.
Conflict of interest statement
Annefleur C. Langedijk, Eline R. Harding, Burak Konya, Bram Vrancken, Robert Jan Lebbink, Anouk Evers, Joukje Willemsen and Philippe Lemey do not report conflicts of interest. Louis J. Bont has not received personal fees or other personal benefits. UMCU has received funding from Abbvie, AstraZeneca, Janssen, the Bill and Melinda Gates Foundation, Nutricia (Danone) and MeMed Diagnostics. UMCU has received major cash or in kind funding as part of the public private partnership IMI‐funded RESCEU project from GSK, Novavax, Janssen, AstraZeneca, Pfizer and Sanofi. UMCU has received major funding by Julius Clinical for participating in the INFORM study sponsored by AstraZeneca. UMCU has received minor funding for participation in trials by Regeneron and Janssen from 2015 to 2017. UMCU received minor funding for consultation and invited lectures by AbbVie, AstraZeneca, Ablynx, Bavaria Nordic, MabXience, Novavax, Pfizer, Janssen. LJB is the founding chairman of the ReSViNET Foundation. Nirsevimab development is jointly funded by AstraZeneca and Sanofi Pasteur.
Figures

Similar articles
-
Genotype Analysis of Respiratory Syncytial Virus Before and After the COVID-19 Pandemic Using Whole-Genome Sequencing: A Prospective, Single-Center Study in Korea From 2019 to 2022.J Korean Med Sci. 2024 Jul 22;39(28):e206. doi: 10.3346/jkms.2024.39.e206. J Korean Med Sci. 2024. PMID: 39048301 Free PMC article.
-
Analysis of respiratory syncytial virus fusion protein from clinical isolates of Korean children in palivizumab era, 2009-2015.J Infect Chemother. 2019 Jul;25(7):514-519. doi: 10.1016/j.jiac.2019.02.013. Epub 2019 Mar 15. J Infect Chemother. 2019. PMID: 30885457
-
Nirsevimab binding-site conservation in respiratory syncytial virus fusion glycoprotein worldwide between 1956 and 2021: an analysis of observational study sequencing data.Lancet Infect Dis. 2023 Jul;23(7):856-866. doi: 10.1016/S1473-3099(23)00062-2. Epub 2023 Mar 17. Lancet Infect Dis. 2023. PMID: 36940703
-
Palivizumab for immunoprophylaxis of respiratory syncytial virus (RSV) bronchiolitis in high-risk infants and young children: a systematic review and additional economic modelling of subgroup analyses.Health Technol Assess. 2011 Jan;15(5):iii-iv, 1-124. doi: 10.3310/hta15050. Health Technol Assess. 2011. PMID: 21281564 Free PMC article. Review.
-
Nirsevimab: review of pharmacology, antiviral activity and emerging clinical experience for respiratory syncytial virus infection in infants.J Antimicrob Chemother. 2023 May 3;78(5):1143-1149. doi: 10.1093/jac/dkad076. J Antimicrob Chemother. 2023. PMID: 36922390 Review.
Cited by
-
Epidemiological and phylogenetic assessment of human respiratory syncytial virus among pediatric patients presenting acute respiratory infections in Shiraz, Iran during 2015-2016.Iran J Microbiol. 2024 Jun;16(3):411-420. doi: 10.18502/ijm.v16i3.15798. Iran J Microbiol. 2024. PMID: 39005603 Free PMC article.
-
Altered RSV Epidemiology and Genetic Diversity Following the COVID-19 Pandemic.Res Sq [Preprint]. 2023 Dec 15:rs.3.rs-3712859. doi: 10.21203/rs.3.rs-3712859/v1. Res Sq. 2023. Update in: Nat Commun. 2024 Apr 20;15(1):3374. doi: 10.1038/s41467-024-47757-9 PMID: 38168164 Free PMC article. Updated. Preprint.
-
Advancing pathogen genomics in resource-limited settings.Cell Genom. 2023 Nov 17;3(12):100443. doi: 10.1016/j.xgen.2023.100443. eCollection 2023 Dec 13. Cell Genom. 2023. PMID: 38116115 Free PMC article. Review.
-
Genomic Epidemiology of Human Respiratory Syncytial Virus, Minnesota, USA, July 2023-February 2024.Emerg Infect Dis. 2024 Nov;30(11):2414-2418. doi: 10.3201/eid3011.241000. Emerg Infect Dis. 2024. PMID: 39447178 Free PMC article.
-
Identification of distinct genotypes in circulating RSV A strains based on variants in the virus replication-associated genes.J Virol. 2024 Aug 20;98(8):e0099024. doi: 10.1128/jvi.00990-24. Epub 2024 Jul 15. J Virol. 2024. PMID: 39007617 Free PMC article.
References
-
- Mazur NI, Higgins D, Nunes MC, et al. The respiratory syncytial virus vaccine landscape: lessons from the graveyard and promising candidates. Lancet Infect Dis. 2018;18(10):e295‐e311. - PubMed
-
- American Academy of Pediatrics Committee on Infectious D, American Academy of Pediatrics Bronchiolitis Guidelines C . Updated guidance for palivizumab prophylaxis among infants and young children at increased risk of hospitalization for respiratory syncytial virus infection. Pediatrics. 2014;134(2):415‐420. - PubMed
-
- PATH. RSV Vaccine and mAb Snapshot . 2020. Accessed March 1, 2021 https://www.path.org/resources/rsv‐vaccine‐and‐mab‐snapshot/
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

