The comparative efficacy and safety of anti-CD20 monoclonal antibodies for relapsing-remitting multiple sclerosis: A network meta-analysis
- PMID: 34505112
- PMCID: PMC8411244
- DOI: 10.1016/j.ibneur.2021.08.003
The comparative efficacy and safety of anti-CD20 monoclonal antibodies for relapsing-remitting multiple sclerosis: A network meta-analysis
Abstract
With the recent successful targeting of B lymphocytes in patients with multiple sclerosis (MS), treatment with anti-CD20 monoclonal antibodies (mAbs) may represent a promising managemental approach, particularly for those with relapsing/remitting MS (RRMS). A network meta-analysis was conducted based on a comprehensive search in Embase, PubMed, and the Cochrane Library to assess the comparative efficacy and safety of currently available anti-CD20 monoclonal antibodies (mAbs), including rituximab, ocrelizumab, and ofatumumab, versus a common comparator (interferon beta-1a [INFβ-1a]) in RRMS patients recruited in randomized clinical trials (RCTs). In a frequentist network meta-analytical model, annualized relapse rates (ARRs) and safety outcomes were expressed as risk ratios (RRs), whereas relapse-free events were expressed as odds ratios (ORs). Treatment ranking was performed using P-scores. The certainty of evidence was appraised using the GRADE approach. Five publications reported the outcomes of seven RCTs (3938 patients, 67.09% females). Compared to INFβ-1a, ocrelizumab reduced the risk of ARR (RR = 0.56, 95% CI, 0.50-0.64), serious adverse events (RR = 0.17, 95% CI, 0.09-0.30), and treatment discontinuation due to adverse events (SAEs, RR = 0.60, 95% CI, 0.39-0.93), and it was associated with higher odds of no relapses (OR = 2.47, 95% CI, 2.00-3.05). Ocrelizumab ranked best among all other treatments in terms of reducing ARR and SAEs. The quality of evidence was low for ocrelizumab, low to moderate for rituximab, and high for ofatumumab. Further large-sized, well-designed RCTs are needed to corroborate the efficacy and safety of ocrelizumab and other anti-CD20 mAbs in RRMS.
Keywords: Monoclonal antibody; Multiple sclerosis; Network meta-analysis; Ocrelizumab; Relapsing-remitting multiple sclerosis.
© 2021 Published by Elsevier Ltd on behalf of International Brain Research Organization.
Conflict of interest statement
None.
Figures
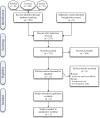


Similar articles
-
Ocrelizumab for multiple sclerosis.Cochrane Database Syst Rev. 2022 May 18;5(5):CD013247. doi: 10.1002/14651858.CD013247.pub2. Cochrane Database Syst Rev. 2022. PMID: 35583174 Free PMC article. Review.
-
Efficacy and safety of monoclonal antibody therapies for relapsing remitting multiple sclerosis: A network meta-analysis.Mult Scler Relat Disord. 2018 Oct;25:322-328. doi: 10.1016/j.msard.2018.08.026. Epub 2018 Aug 29. Mult Scler Relat Disord. 2018. PMID: 30195200
-
Systematic review and network meta-analysis comparing ocrelizumab with other treatments for relapsing multiple sclerosis.Mult Scler Relat Disord. 2019 Apr;29:55-61. doi: 10.1016/j.msard.2018.12.040. Epub 2019 Jan 2. Mult Scler Relat Disord. 2019. PMID: 30677733
-
Immunomodulators and immunosuppressants for multiple sclerosis: a network meta-analysis.Cochrane Database Syst Rev. 2013 Jun 6;(6):CD008933. doi: 10.1002/14651858.CD008933.pub2. Cochrane Database Syst Rev. 2013. PMID: 23744561 Review.
-
Comparative safety of high-efficacy disease-modifying therapies in relapsing-remitting multiple sclerosis: a systematic review and network meta-analysis.Neurol Sci. 2022 Sep;43(9):5479-5500. doi: 10.1007/s10072-022-06197-3. Epub 2022 Jun 17. Neurol Sci. 2022. PMID: 35713731
Cited by
-
Five-year efficacy outcomes of ocrelizumab in relapsing multiple sclerosis: A propensity-matched comparison of the OPERA studies with other disease-modifying therapies in real-world lines of treatments.J Cent Nerv Syst Dis. 2024 Sep 13;16:11795735241260563. doi: 10.1177/11795735241260563. eCollection 2024. J Cent Nerv Syst Dis. 2024. PMID: 39290861 Free PMC article.
-
Impact of Anti-CD20 therapies on the immune homeostasis of gastrointestinal mucosa and their relationship with de novo intestinal bowel disease in multiple sclerosis: a review.Front Pharmacol. 2023 May 30;14:1186016. doi: 10.3389/fphar.2023.1186016. eCollection 2023. Front Pharmacol. 2023. PMID: 37324473 Free PMC article. Review.
-
The role of non-coding RNAs in neuroinflammatory process in multiple sclerosis.Mol Neurobiol. 2022 Aug;59(8):4651-4668. doi: 10.1007/s12035-022-02854-y. Epub 2022 May 19. Mol Neurobiol. 2022. PMID: 35589919 Review.
References
-
- Bar-Or A., Grove R.A., Austin D.J., Tolson J.M., Vanmeter S.A., Lewis E.W., Derosier F.J., Lopez M.C., Kavanagh S.T., Miller A.E., Sorensen P.S. Subcutaneous ofatumumab in patients with relapsing-remitting multiple sclerosis: the MIRROR study. Neurology. 2018;90:e1805–e1814. doi: 10.1212/WNL.0000000000005516. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
